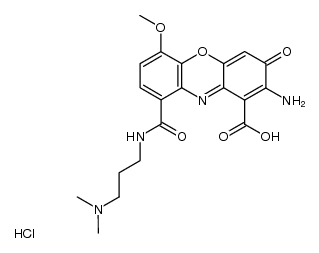
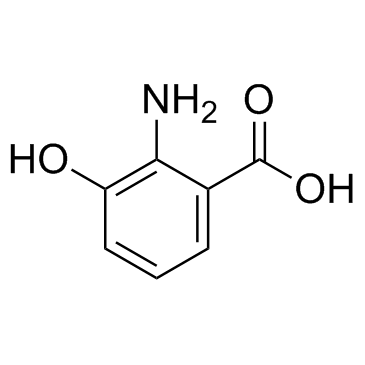
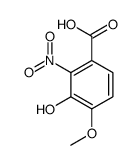
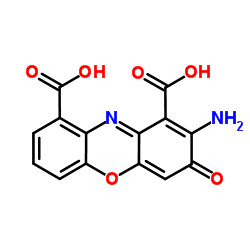
606-59-7
| Name | Cinnabarinic Acid |
|---|---|
| Synonyms |
2-amino-3-oxophenoxazine-1,9-dicarboxylic acid
2-Amino-3-oxo-3H-phenoxazine-1,9-dicarboxylic acid |
| Description | Cinnabarinic acid is a specific orthosteric agonist of mGlu4 by interacting with residues of the glutamate binding pocket of mGlu4, has no activity at other mGlu receptors. Cinnabarinic acid is an endogenous metabolite of the kynurenine pathway of tryptophan. Cinnabarinic acid induces cell apoptosis[1]. |
|---|---|
| Related Catalog | |
| Target |
mGluR4 |
| In Vitro | Cinnabarinic acid (0-100 μM) does not activate mGlu1, mGlu2, mGlu5, mGlu6, mGlu7, and mGlu8 receptors as shown by measurements of [3H]InsP formation. In contrast, cinnabarinic acid acts as a partial agonist of mGlu4 receptors by increasing [3H]InsP formation by approximately 35% at 100 μM, which is 5-fold less efficacious than ACPT-I in activating mGlu4 receptors in HEK293 cells transiently transfected with rat mGlu1, -2, -4, -5, -6, -7, or -8 receptors[1]. Cinnabarinic acid (0-100 μM) reduces cAMP formation in a concentration-dependent manner with an excellent potency and efficacy. At 30 μM, cinnabarinic acid is effective at 30 μM, and substantially inhibits cAMP formation in cultured cerebellar granule cells[1]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 536.8±50.0 °C at 760 mmHg |
| Melting Point | >300ºC |
| Molecular Formula | C14H8N2O6 |
| Molecular Weight | 300.223 |
| Flash Point | 278.4±30.1 °C |
| Exact Mass | 300.038239 |
| PSA | 143.72000 |
| LogP | -0.13 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.780 |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
606-59-7 |
| Literature: Korshunova, Z. I.; Popova, E. B.; Glibin, E. N.; Ginzburg, O. F. Journal of Organic Chemistry USSR (English Translation), 1991 , vol. 27, # 2.2 p. 314 - 319 Zhurnal Organicheskoi Khimii, 1991 , vol. 27, # 2 p. 369 - 376 |
|
~% 
606-59-7 |
| Literature: Korshunova, Z. I.; Popova, E. B.; Glibin, E. N.; Ginzburg, O. F. Journal of Organic Chemistry USSR (English Translation), 1991 , vol. 27, # 2.2 p. 314 - 319 Zhurnal Organicheskoi Khimii, 1991 , vol. 27, # 2 p. 369 - 376 |
|
~% 
606-59-7 |
| Literature: Manthey, Michael K.; Pyne, Stephen G.; Truscott, Roger J. W. Journal of Organic Chemistry, 1988 , vol. 53, # 7 p. 1486 - 1488 |
|
~% 
606-59-7
Detail
|
| Literature: Korshunova, Z. I.; Popova, E. B.; Glibin, E. N.; Ginzburg, O. F. Journal of Organic Chemistry USSR (English Translation), 1991 , vol. 27, # 2.2 p. 314 - 319 Zhurnal Organicheskoi Khimii, 1991 , vol. 27, # 2 p. 369 - 376 |
|
~% 
606-59-7
Detail
|
| Literature: Korshunova, Z. I.; Popova, E. B.; Glibin, E. N.; Ginzburg, O. F. Journal of Organic Chemistry USSR (English Translation), 1991 , vol. 27, # 2.2 p. 314 - 319 Zhurnal Organicheskoi Khimii, 1991 , vol. 27, # 2 p. 369 - 376 |
|
~% 
606-59-7 |
| Literature: Butenandt et al. Justus Liebigs Annalen der Chemie, 1957 , vol. 602, p. 72,79 |
|
~% 
606-59-7
Detail
|
| Literature: Hick, Larry A.; Manthey, Michael K.; Truscott, Roger J. W. Journal of Heterocyclic Chemistry, 1991 , vol. 28, # 4 p. 1157 - 1160 |
|
~38% 
606-59-7 |
| Literature: Pasceri, Raffaele; Siegel, David; Ross, David; Moody, Christopher J. Journal of Medicinal Chemistry, 2013 , vol. 56, # 8 p. 3310 - 3317 |
|
~% 
606-59-7
Detail
|
| Literature: Korshunova, Z. I.; Popova, E. B.; Glibin, E. N.; Ginzburg, O. F. Journal of Organic Chemistry USSR (English Translation), 1991 , vol. 27, # 2.2 p. 314 - 319 Zhurnal Organicheskoi Khimii, 1991 , vol. 27, # 2 p. 369 - 376 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |