1234490-83-5
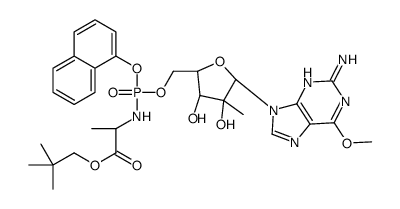
| Name | 2,2-dimethylpropyl (2S)-2-[[[(2R,3R,4R,5R)-5-(2-amino-6-methoxypurin-9-yl)-3,4-dihydroxy-4-methyloxolan-2-yl]methoxy-naphthalen-1-yloxyphosphoryl]amino]propanoate |
|---|---|
| Synonyms |
unii-62f4ad749y
inx-189 INX-08189 |
| Description | BMS-986094 is a potent inhibitor of hepatitis C virus (HCV) replication, with an EC50 of 35 nM at 24 h in Huh-7 cells. BMS-986094 is a phosphoramidate prodrug of 6-O-methyl-2’-C-methyl guanosine, and can be researched for chronic HCV infection[1][2]. |
|---|---|
| Related Catalog | |
| Target |
EC50: 35 nM (HCV)[1] |
| In Vitro | BMS-986094 (INX-08189) is a highly potent inhibitor of HCV replication, with the EC50s of 10 nM against genotype 1b, 12 nM against genotype 1a, and 0.9 nM against genotype 2a after 72 h of exposure. And the concentration resulting in 50% cellular cytotoxicity (CC50) in cultured Huh-7 cells is 7.01 μM[1]. BMS-986094 (5-80 nM; 14 days) decreases luciferase activity in a concentration-dependent manner in genotype 1b replicon cells[1]. BMS-986094 (20 μM; 3 days ) decreases relative mitochondrial copy number of 11% in CEM cells. BMS-986094 (1 μM; 14 days ) has no effect on mitochondrial copy number in CEM cells. BMS-986094 does not alter the relative mitochondrial copy number in HepG2 cells[1]. MS-986094 (10 µM; 24 hours) does not increase apparently in the concentration of BMS-986094 or its metabolites in human hepatocytes (HHs) and human cardiomyocytes (HCMs) except that intracellular concentrations of INX-09114 increases and plateaues after a 7-hour incubation in HCM[3]. |
| In Vivo | BMS-986094 (3-300 mg/kg; p.o.) converts to 2′-C-Me-GTP after oral administration, and 2’-C-MeG in the plasma is proportional to the production of 2’-C-MeGTP in the liver[1]. BMS-986094 (25 mg/kg; p.o.) is efficiently extracts from the portal circulation by the liver following oral administration in cynomolgus monkeys[1]. BMS-986094 (15 or 30 mg/kg/d; p.o. for 3 weeks) administers cynomolgus monkeys, the nucleoside metabolite M2 was the major plasma analyte, and INX-09114 was the highest drug-related species in the heart and kidney[3]. Animal Model: Male Sprague-Dawley rats[1] Dosage: 3, 5, 10, 25 mg/kg Administration: A single p.o. administration Result: At doses of ≥5 mg/kg, the concentrations of 2′-C-MeGTP in the liver exceeded the EC90 soon after dosing and remained at or above this level for 72 h. |
| References |
| Density | 1.47 |
|---|---|
| Molecular Formula | C30H39N6O9P |
| Molecular Weight | 658.63900 |
| Exact Mass | 658.25200 |
| PSA | 212.94000 |
| LogP | 3.67170 |