86408-36-8
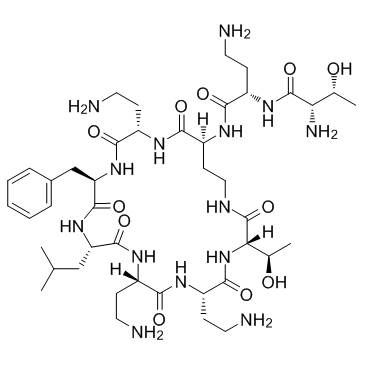
| Name | (2S,3R)-2-amino-N-[(2S)-4-amino-1-oxo-1-[[(3S,6S,9S,12S,15R,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-(1-hydroxyethyl)-12-(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]-3-hydroxybutanamide |
|---|---|
| Synonyms |
triisopropylsilanthiol
H-Thr-2,4-diaminobutyryl-cyclo[2,4-diaminobutyryl-2,4-diaminobutyryl-D-Phe-Leu-2,4-diaminobutyryl-2,4-diaminobutyryl-Thr] polymyxin B nonapeptide HSSi(i-Pr)3 TRI-ISOPROPYLSILANETHIOL H-Thr-Dab-cyclic-(Dab-Dab-D-Phe-Leu-Dab-Dab-Thr) polymixin B nonapeptide T-X-cyclo[X-X-DF-L-X-X-T] |
| Description | Polymyxin B nonapeptide is a cyclic peptide obtained from Polymyxin B by proteolytic removal of its terminal amino acyl residue[1]. Polymyxin B nonapeptide is less toxic, lacks bactericidal activity, and retains its ability to render gram-negative bacteria susceptible to several antibiotics by permeabilizing their outer membranes[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Polymyxin B nonapeptide, a cationic cyclic peptide derived by enzymatic processing from the naturally occurring peptide polymyxin B, is able to increase the permeability of the outer membrane of Gram-negative bacteria toward hydrophobic antibiotics probably by binding to the bacterial lipopolysaccharide (LPS)[1]. |
| References |
| Density | 1.32g/cm3 |
|---|---|
| Boiling Point | 1456.2ºC at 760 mmHg |
| Molecular Formula | C43H74N14O11 |
| Molecular Weight | 963.13500 |
| Flash Point | 834.5ºC |
| Exact Mass | 962.56600 |
| PSA | 463.87000 |
| LogP | 0.27100 |
| Index of Refraction | 1.607 |
| Storage condition | 2-8℃ |