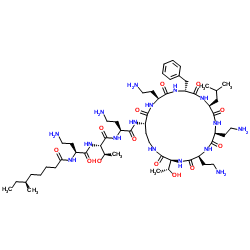
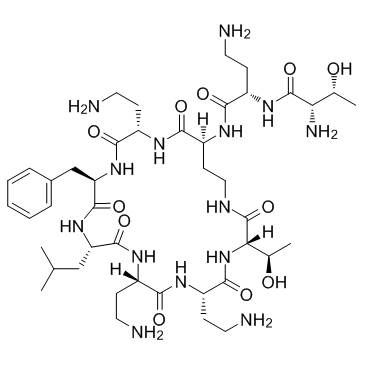
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TR1055000
-
CHEMICAL NAME :
-
Polymyxin B1
-
CAS REGISTRY NUMBER :
-
4135-11-9
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
3
-
MOLECULAR FORMULA :
-
C56-H98-N16-O13
-
MOLECULAR WEIGHT :
-
1203.70
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
19 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GDA2 "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980- Volume(issue)/page/year: 4(1),334,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
80 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GDA2 "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980- Volume(issue)/page/year: 4(1),334,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GDA2 "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980- Volume(issue)/page/year: 4(1),334,1980
|