1255204-84-2
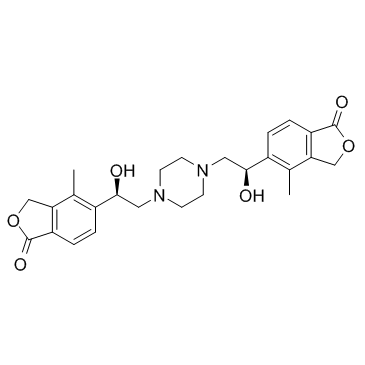
| Name | 5-[(1R)-1-hydroxy-2-[4-[(2R)-2-hydroxy-2-(4-methyl-1-oxo-3H-2-benzofuran-5-yl)ethyl]piperazin-1-yl]ethyl]-4-methyl-3H-2-benzofuran-1-one |
|---|---|
| Synonyms |
5,5'-[piperazine-1,4-diylbis(1-hydroxyethane-2,1-diyl)]bis(4-methyl-2-benzofuran-1(3H)-one)
MK-7145 5,5'-((1R,1'R)-Piperazine-1,4-diylbis(1-hydroxyethane-2,1-diyl))bis(4-methylisobenzofuran-1(3H)-one) 1(3H)-Isobenzofuranone,5,5'-(1,4-piperazinediylbis((1R)-1-hydroxy-2,1-ethanediyl))bis(4-methyl |
| Description | MK-7145 is a ROMK inhibitor, with an IC50 of 0.045 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.045 μM (ROMK)[1]. |
| In Vitro | MK-7145 (Compound 12) is screened against other members of the Kir family of channels. It doed not show any significant activity on Kir2.1, Kir2.3, Kir4.1, or Kir7.1 channels when tested at concentrations up to 30 μM. MK-7145 is also selective against other cardiac ion channels such as Cav1.2 and Nav1.5 (IC50>30 μM). In a broad counterscreen panel conducted at Panlabs and including over 150 receptors, enzymes, and ion channels, MK-7145 only exhibits three activities at <10 μM: acetyl cholinesterase, ACES, IC50=9.94 μM, somatostatin subtype 1, sst1 IC50=2.63 μM, and human serotonin transporter, SERT, IC50=0.12 μM. In a functional assay using HEK293 cells stably transfected with human SERT, uptake of 3H-serotonin is inhibited by MK-7145 with an IC50 value of 2.40±0.32 μM (n=5). The superior ROMK potency and in vivo efficacy of MK-7145, coupled with the fact that MK-7145 is a substrate of human Pgp (human Mdr1 BAAB ratio=12), should be able to impart a significant safety window with respect to the SERT off-target activity. |
| References |
| Molecular Formula | C26H30N2O6 |
|---|---|
| Molecular Weight | 466.52600 |
| Exact Mass | 466.21000 |
| PSA | 99.54000 |
| LogP | 1.90460 |
|
~% 
1255204-84-2 |
| Literature: MERCK SHARP andamp;; DOHME CORP.; PASTERNAK, Alexander; SHAHRIPOUR, Aurash; TANG, Haifeng; TEUMELSAN, Nardos, H.; YANG, Lihu; ZHU, Yuping; WALSH, Shawn, P. Patent: WO2010/129379 A1, 2010 ; Location in patent: Page/Page column 105-106 ; WO 2010/129379 A1 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |