314728-85-3
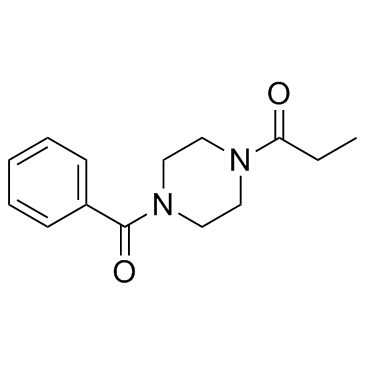
| Name | 1-(4-benzoylpiperazin-1-yl)propan-1-one |
|---|---|
| Synonyms |
1-(4-Benzoyl-1-piperazinyl)-1-propanone
1-(4-Benzoylpiperazin-1-yl)propan-1-one Lopac-D-5689 DM 235 DM-235 Sunifiram |
| Description | Sunifiram (DM-235) is a piperazine derived ampakine-like drug which has nootropic effects in animal studies with significantly higher potency than piracetam.IC50 value: Target: in vitro: DM 232 and DM 235 are novel antiamnesic compounds structurally related to ampakines. The involvement of AMPA receptors in the mechanism of action of DM 232 and DM 235 was, therefore, investigated in vivo and in vitro. Both compounds (0.1 mg/kg i.p.) were able to reverse the amnesia induced by the AMPA receptor antagonist NBQX (30 mg/kg i.p.) in the mouse passive avoidance test. At the effective doses, the investigated compounds did not impair motor coordination, as revealed by the rota rod test, nor modify spontaneous motility and inspection activity, as revealed by the hole board test [1]. In mouse hippocampal slices, sunifiram at 10-100 nM significantly enhanced LTP in a bell-shaped dose-response relationship which peaked at 10 nM. The enhancement of LTP by sunifiram treatment was inhibited by 7-chloro-kynurenic acid (7-ClKN), an antagonist for glycine-binding site of NMDAR, but not by ifenprodil, an inhibitor for polyamine site of NMDAR [2].in vivo: OBX mice were administered once a day for 7-12 days with sunifiram (0.01-1.0 mg/kg p.o.) from 10 days after operation with or without gavestinel (10 mg/kg i.p.), which is glycine-binding site inhibitor of N-methyl-d-aspartate receptor (NMDAR) [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.0±38.0 °C at 760 mmHg |
| Molecular Formula | C14H18N2O2 |
| Molecular Weight | 246.305 |
| Flash Point | 205.0±19.1 °C |
| Exact Mass | 246.136826 |
| PSA | 40.62000 |
| LogP | 0.26 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.561 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |