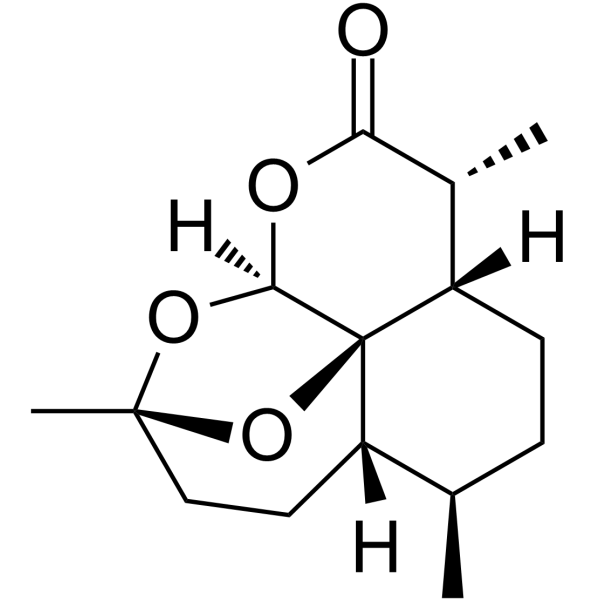
63968-64-9
| Name | (+)-artemisinin |
|---|---|
| Synonyms |
(3R,5aS,6R,8aS,9R,12S,12aR)-3,6,9-trimethyloctahydro-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10(3H)-one
(4S,5R,8S,9R,12S,13R)-1,5,9-Trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0.0]hexadecan-10-one artemisininum qinghausu Artemisinin UNII-9RMU91N5K2 ARTEANUIN Quinghaosu [3R-(3R,5aS,6S,8aS,9R,10R,12S,12aR**)]-Decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-one qinghaosu QINGHAOSA artemisinine (+)-artemisinin MFCD00081057 [3R-(3a,5ab,6b,8ab,9a,12b,12aR*)]-Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10(3H)-one qinghausau ARTEMISINE QHS qinghosu Arteannuin |
| Description | Artemisinin is an anti-malarial drug isolated from the aerial parts of Artemisia annua L. plants. |
|---|---|
| Related Catalog | |
| In Vitro | Artemisinin (3.125-100 μM) concentration-dependently suppresses Aβ25-35 induced cytotoxicity in PC12 cells. Artemisinin (25 μM) suppresses Aβ25-35-induced LDH release, apoptosis and ROS production, attenuates Aβ-induced mitochondrial membrane potential loss and caspase 3/7 activity increase, and stimulates the phosphorylation of ERK1/2 in a time- and concentration-dependent manner in PC12 cell. ERK 1/2 pathway mediates the protect effects of artemisinin in PC12 cells[1]. Artemisinin shows cytotoxic activity in MCF-7/Dox cell line with IC50 of 3.7±0.4 μg/mL after 24 h treatment. Besides, Artemisinin treatment of MCF-7 cells, sensitive and resistant to Dox and DDP, causes a decrease in expression of proteins such as LF, FTH1, and HEP. Artemisinin activates p38 MAPK-kinase cascade regardless of the oxidative stress due to inhibition of VEGF expression and cell migration[2]. Artemisinin (50, 100 or 200 mg) significantly inhibits isoflurane-induced hippocampal neuronal loss, decreases caspase-3-positive cell counts and also cleaves caspase-3 expression, and modulates the expression of apoptosis pathway proteins. Artemisinin modulates JNK/ERK 1/2 signalling and histone acetylation[3]. Artemisinin inhibits HCV replication in a dose-dependent manner with EC50 value of 167±38 µM. Artemisinin and its most potent analogues partially inhibit the in vitro replication of HCV by induction of reactive oxygen species (ROS)[4]. Artemisinin significantly inhibits VSMC proliferation in a dose-dependent manner. Artemisinin (1 mM) for 72 h significantly reduces the expression of proliferating cell nuclear antigen messenger RNA[5]. |
| In Vivo | Artemisinin (50, 100 or 200 mg/kg b.wt/day, p.o.) prevents isoflurane-induced working memory impairments as observed in T-maze test. Artemisinin enhances spatial navigation and memory of rats exposed to isoflurane. Artemisinin-treated rats exhibit markedly better performance in comparison with isoflurane-alone-exposed rats[3]. |
| Kinase Assay | Briefly, PC12 cells are lysed in lysis buffer and centrifuged at 12,500×g for 5 min 15 mL of cell lysate is incubated with 50 mL of 2X substrate working solution at room temperature for 30 min in 96-well plates. The fluorescence intensity is then determined by Infinite M200 PRO Multimode Microplate at an excitation wavelength of 490 nm and emission at 520 nm. The fluorescence intensity of each sample is normalized to the protein concentration of sample. All values of % caspase 3/7 activities are normalized to the control group. |
| Cell Assay | For this purpose, cells are cultivated in 96-well plates in DMEM, supplemented with insulin. The artemisinin, Dox, and DDP are added to media at different concentrations and the cells are cultivated for either 24 or 48 h. For this purpose artemisinin is diluted in 0.01% DMSO in media. After this time, 10 µL of the MTT dye solution (5 mg/mL in phosphate buffer saline) is added to the cells; the cells are incubated at the same conditions for 3 h. After centrifugation (1500 rpm, 5 min) the supernatant is removed. 100 μL of dimethyl sulfoxide is added to each well, to dissolve formazan. The absorption is measured, using a multi-well spectrophotometer at a wavelength of 540 nm. |
| Animal Admin | Separate group of rat pups (total rat pups 80; n = 16 per group) is administered artemisinin (50, 100 or 200 mg/kg body weight) via oral gavage, every day from P2 to P21. On P7, the pups are exposed to isoflurane (0.75% in 30% oxygen or air) for 6 h in a temperature-controlled chamber. Animals that are not exposed to anaesthesia nor given artemisinin served as control group, while rats that receive isoflurane alone are grouped as anaesthetic-controls. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 389.9±42.0 °C at 760 mmHg |
| Melting Point | 156-157ºC |
| Molecular Formula | C15H22O5 |
| Molecular Weight | 282.332 |
| Flash Point | 172.0±27.9 °C |
| Exact Mass | 282.146729 |
| PSA | 53.99000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.533 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KD4170000 |
| HS Code | 2932999099 |
| Precursor 4 | |
|---|---|
| DownStream 6 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



![2,5,5-trimethyl-2-[2'-((1''S)-2''-formyl-6''(R)-methylcyclohex-2''-enyl)ethyl]-1,3-dioxane structure](https://image.chemsrc.com/caspic/129/116399-90-7.png)
![2,5,5-trimethyl-2-[2'-[1''R-methyl-3''-[(trimethylsilyl)hydroxymethyl]cyclohex-3''-en-2''-yl]ethyl]-1,3-dioxane acetyl ester structure](https://image.chemsrc.com/caspic/091/116399-91-8.png)


![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin structure](https://image.chemsrc.com/caspic/076/82534-75-6.png)