175865-59-5
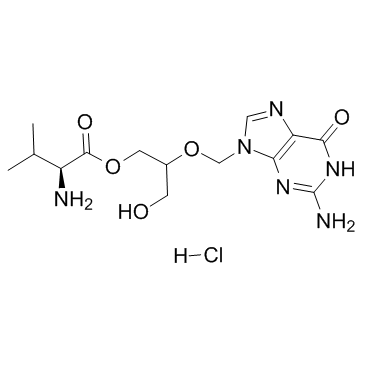
| Name | Valganciclovir hydrochloride |
|---|---|
| Synonyms |
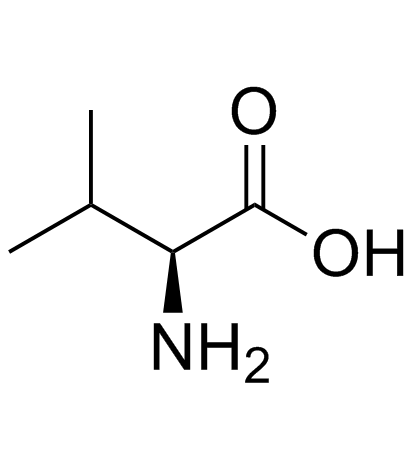
(2S)-2-amino-3-méthylbutanoate de 2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)méthoxy]-3-hydroxypropyle chlorhydrate
L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, hydrochloride (1:1) L-valine, 2-[(2-amino-3,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, monohydrochloride 2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl L-valinate hydrochloride 2-[(2-Amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl-(2S)-2-amino-3-methylbutanoathydrochlorid Valganciclovir Hydrochloride Valganciclovir HCl 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl L-valinate hydrochloride (1:1) L-Valine, 2-[(2-amino-3,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, hydrochloride (1:1) 2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl (2S)-2-amino-3-methylbutanoate hydrochloride L-Valine (2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester Valganciclovir (hydrochloride) |
| Description | Valganciclovir (hydrochloride), the L-valyl ester of ganciclovir, is actually a prodrug for ganciclovir. Valganciclovir is an antiviral medication used to treat cytomegalovirus infections.IC50 Value: Target: CMVin vitro: In cell culture model systems using Caco-2 cells for PEPT1 and SKPT cells for PEPT2, valganciclovir inhibited glycylsarcosine transport mediated by PEPT1 and PEPT2 with K(i) values (inhibition constant) of 1.68+/-0.30 and 0.043+/- 0.005 mM, respectively. The inhibition by valganciclovir was competitive in both cases [1].in vivo: 37 patients were enrolled; 19 patients received treatment with VGV and 18 patients received treatment with GCV. The VGV was not inferior in efficacy to GCV as pre-emptive therapy, with rates of viral clearance at 28 days of 89.5% and 83%, respectively (P-value for non-inferiority = 0.030). Toxicities were similar between the 2 arms. No patients developed CMV disease [2]. Patients being treated with an alemtuzumab-containing regimen received prophylaxis with either valaciclovir 500 mg orally daily orvalganciclovir 450 mg orally twice daily. None of the 20 patients randomized to valganciclovir experienced CMV reactivation (P = .004) [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 629.1ºC at 760 mmHg |
|---|---|
| Molecular Formula | C14H23ClN6O5 |
| Molecular Weight | 390.823 |
| Exact Mass | 390.141846 |
| PSA | 171.37000 |
| LogP | 0.64680 |
| Vapour Pressure | 1.08E-16mmHg at 25°C |
| Storage condition | -20°C |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 8 | |
|---|---|
| DownStream 0 | |



![2-[(2-amino-1,6-dihydro-6-oxopurin-9-yl)methyloxy]-3-hydroxypropyl 2'-(S)-azido-3'-methylbutanoate structure](https://image.chemsrc.com/caspic/245/1219792-38-7.png)
![6H-Purin-6-one, 1,9-dihydro-9-[[1-(hydroxyMethyl)-2-(triphenylmethoxy)ethoxy]Methyl]-2-[(triphenylmethyl)amino] structure](https://image.chemsrc.com/caspic/102/109082-85-1.png)