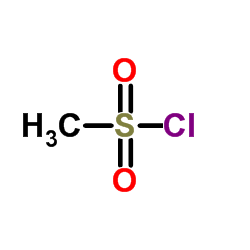
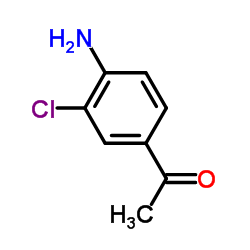
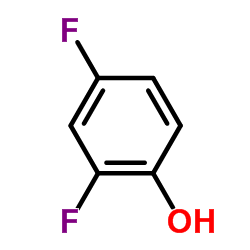
116686-15-8
| Name | N-[4-acetyl-2-(2,4-difluorophenoxy)phenyl]methanesulfonamide |
|---|---|
| Synonyms |
N-(4-Acetyl-2-(2,4-difluorophenoxy)phenyl)methanesulfonamide
N-[4-Acetyl-2-(2,4-difluorophenoxy)phenyl]methanesulfonamide fk 3311 cox-2 inhibitor v |
| Description | FK 3311 is a selective inhibitor of COX-2; antiinflammatory agent.IC50 Value: 1.6 uM [1]Target: COX-2Cyclooxygenase (COX) is an intracellular enzyme that converts arachidonic acid into prostaglandin (PG)G2 and PGH2.in vitro: The racemic mixtures and the (R)- and (S)-isomers of the 2 metabolites were inactive in the PGE2 test. IC50 values were more than 100 uM for (2 and 5), compared to 1.6 uM for FK-3311. Antiinflammatory activity was assessed by inhibition of adjuvant-induced arthritis, and analgesic activity was determined in the acetic acid-induced writhing assay. Following p.o. administration of 10 mg/kg, racemic (2) and its optical isomers showed activity comparable to FK-3311 (76% inhibition) in the adjuvant arthritis test, whereas racemic (5) showed very weak activity, and (R)- and (S)-(5) were not tested. With regard to analgesic effects, FK-3311 and racemic (2) showed 81 and 62% inhibitions, respectively, at a dose of 100 mg/kg p.o. The (R)- and (S)-isomers of (2) and racemic (5) all showed 46% inhibition of writhing syndrome. (R)- and (S)-(5) were less active showing 16 and 20% inhibitions, respectively[1].in vivo: L-PVR, CO, PaO(2), and WDR were significantly (P < 0.05) better in the FK group than in the control group. Histological tissue edema was mild, and PMN infiltration was significantly (P < 0.05) reduced in the FK group compared to the control group. The serum TxB(2) levels were significantly (P < 0.05) lower in the FK group than in the control group, while 6-keto-PGF(1alpha) levels were not significantly (P < 0.05) reduced. Two-day survival rate was significantly (P < 0.05) better in the FK group than in the control group [2]. Survival rate was significantly better (p <. 05) and serum GOT levels 30 min after reperfusion were significantly lower (p <. 05) in the FK high-dose group compared to the other two groups. Four hours after reperfusion, GPT levels and liver tissue flow were significantly (p <. 05) better in the FK high-dose group compared to the control. Both 30 min and 4 hr after reperfusion, serum TxB(2) levels were significantly lower in the FK high-dose group compared to the control (p <. 05) [3]. |
|---|---|
| Related Catalog | |
| Target |
COX-2 |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 431.3±55.0 °C at 760 mmHg |
| Molecular Formula | C15H13F2NO4S |
| Molecular Weight | 341.330 |
| Flash Point | 214.6±31.5 °C |
| Exact Mass | 341.053345 |
| PSA | 80.85000 |
| LogP | 3.22 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.579 |
| Storage condition | 2-8℃ |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
|
~% 
116686-15-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2399 - 2409 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |