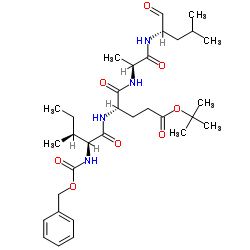
158442-41-2
| Name | N-[(Phenylmethoxy)carbonyl]-L-isoleucyl-L-α-glutamyl-tert-butylester-N-[(1S)-1-formyl-3-methylbutyl]-L-alaninamide |
|---|---|
| Synonyms |
tert-Butyl (4S)-4-{[(2S,3S)-2-{[(benzyloxy)carbonyl]amino}-3-methylpentanoyl]amino}-5-{[(2S)-1-{[(2S)-4-methyl-1-oxopentan-2-yl]amino}-1-oxopropan-2-yl]amino}-5-oxopentanoate (non-preferred name)
PROTEASOME INHIBITOR I M.W. 618.77 C32H50N4O8 N-Cb 2-Methyl-2-propanyl (4S)-4-{[(2S,3S)-2-{[(benzyloxy)carbonyl]amino}-3-methylpentanoyl]amino}-5-{[(2S)-1-{[(2S)-4-methyl-1-oxo-2-pentanyl]amino}-1-oxo-2-propanyl]amino}-5-oxopentanoate (non-preferred name) |
| Description | PSI (Proteasome Inhibitor 1) is a potent proteasome inhibitor. PSI inhibits the proliferation of primary effusion lymphoma (PEL) cells. PSI has the potential for the research of Kaposi’s sarcoma-associated herpesvirus (KSHV) infection and KSHV-associated lymphomas[1]. |
|---|---|
| Related Catalog | |
| In Vitro | PSI (24 h) inhibits the proliferation of primary effusion lymphoma (PEL) cells at low nanomolar concentrations (CC50s of 205, 190, 22.0, 53.0 nM FOR BJAB, Ramos, BC3, BCBL1 cells, respectively)[1]. PSI (50 nM; 6 h) increases caspase-3/7 activity by 8-fold compared with control[1]. PSI (50 nM; 6 h) decreases the transcriptional activity of NF-κB by 52%[1]. PSI (1, 5 nM; 3 days) inhibits the growth of BC3 cells at a high concentration (5 nM)[1]. Cell Cytotoxicity Assay[1] Cell Line: BC3, BCBL1, Ramos, BJAB cells Concentration: Incubation Time: 24 h Result: Inhibited the proliferation of primary effusion lymphoma (PEL) cells at low nanomolar concentrations (CC50s of 205, 190, 22.0, 53.0 nM FOR BJAB, Ramos, BC3, BCBL1 cells, respectively). Western Blot Analysis[1] Cell Line: HBL6 cells Concentration: 50 nM Incubation Time: 6 h Result: Decreased the NF-κB activity by 52%. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 844.2±65.0 °C at 760 mmHg |
| Molecular Formula | C32H50N4O8 |
| Molecular Weight | 618.761 |
| Flash Point | 464.4±34.3 °C |
| Exact Mass | 618.362854 |
| PSA | 169.00000 |
| LogP | 5.79 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.510 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |