120791-76-6
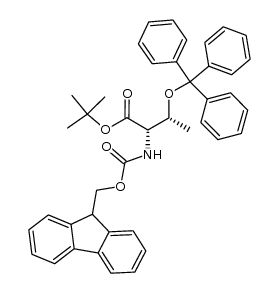
| Name | tert-butyl (2S,3R)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-hydroxybutanoate |
|---|---|
| Synonyms |
Nalpha-Fmoc-L-threonine tert-Butyl Ester
Fmoc-L-threonine tert-butyl ester Nα-Fmoc-L-threonine tert-Butyl Ester N-fluorenylmethoxycarbonyl-threonine-tert-butyl ester Fmoc-Thr-OtBu Fmoc-threonine tert-butyl ester Nα-[(9H-Fluoren-9-ylMethoxy)carbonyl]-L-threonine tert-Butyl Ester Fmoc-Thr-tBu |
| Description | Fmoc-Thr-OBu-t is a threonine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | 100 ℃ |
|---|---|
| Molecular Formula | C23H27NO5 |
| Molecular Weight | 397.46400 |
| Exact Mass | 397.18900 |
| PSA | 84.86000 |
| LogP | 4.00710 |
| Storage condition | Refrigerator |
|
~% 
120791-76-6 |
| Literature: Journal of Organic Chemistry, , vol. 68, # 17 p. 6795 - 6798 |
|
~% 
120791-76-6 |
| Literature: Liebigs Annalen der Chemie, , p. 751 - 770 |
|
~% 
120791-76-6 |
| Literature: Liebigs Annalen der Chemie, , p. 751 - 770 |
|
~% 
120791-76-6 |
| Literature: Liebigs Annalen der Chemie, , p. 751 - 770 |
|
~% 
120791-76-6 |
| Literature: European Journal of Organic Chemistry, , # 20-21 p. 3685 - 3689 |
|
~94% 
120791-76-6 |
| Literature: Journal of Organic Chemistry, , vol. 68, # 17 p. 6795 - 6798 |
|
~77% 
120791-76-6 |
| Literature: Angewandte Chemie (International Edition in English), , vol. 36, # 6 p. 618 - 621 |
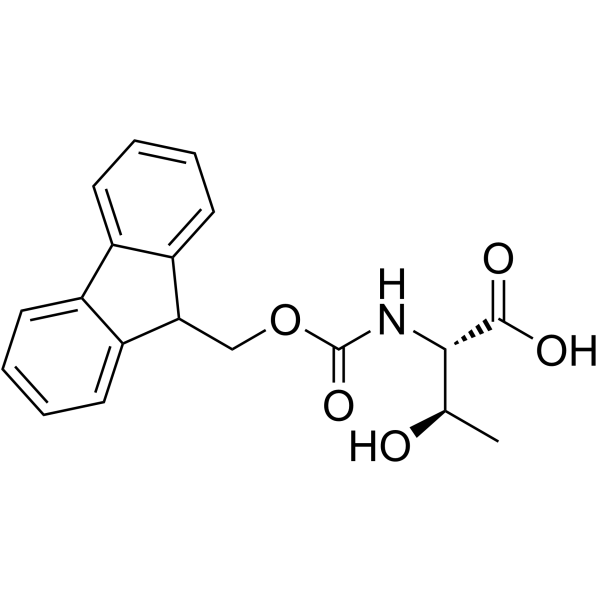
| Precursor 8 | |
|---|---|
| DownStream 1 | |

![Fmoc-Thr[GalNAc(Ac)3-α-D]-OH structure](https://image.chemsrc.com/caspic/350/116783-35-8.png)