1802175-06-9
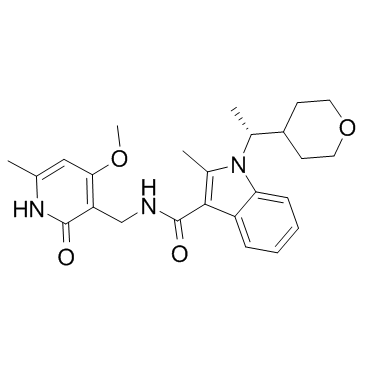
| Name | N-[(4-Methoxy-6-methyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-2-methyl-1-[(1R)-1-(tetrahydro-2H-pyran-4-yl)ethyl]-1H-indole-3-carboxamide |
|---|---|
| Synonyms |
N-[(4-Methoxy-6-methyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-2-methyl-1-[(1R)-1-(tetrahydro-2H-pyran-4-yl)ethyl]-1H-indole-3-carboxamide
CPI-360 |
| Description | CPI-360 is a potent, selective EZH2inhibitor with IC50 of 0.5 nM and 2.5 nM nM for wt EZH2 and Y641N EZH2, respectively.IC50 value: 0.5 nM, 2.5 nMTarget: EZH2in vitro: CPI-360 functions on the basis of S-adenosyl-Lmethionine(SAM)-competition, inhibits EZH1 about 100-fold less and shows exquisite selectivity across a large panel of histone lysine and arginine, and DNA methyltransferases. CPI-360 potently reduced global H3K27me3 and H3K27me2 levels in a dosedependent manner. CPI-360 effectively suppressed heavy H3K27me3 incorporation in KARPAS-422 cells without affecting total histone turnover. CPI-360 treatment causes time-dependent transcriptional changes in germinal center B cell-like diffuse large B cell lymphoma.in vivo: Twice daily, subcutaneous administration of 200 mg/kg ofCPI-360 reduced tumor growth (TGI 44%) of KARPAS-422 xenografts in mice without affecting body weight or causing any overt adverse effects. CPI-360 completely inhibits EZH2 catalytic activity, since we entirely suppress H3K27me3 turnover over time. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 712.2±60.0 °C at 760 mmHg |
| Molecular Formula | C25H31N3O4 |
| Molecular Weight | 437.531 |
| Flash Point | 384.5±32.9 °C |
| Exact Mass | 437.231445 |
| LogP | 2.20 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.621 |
| Storage condition | -20℃ |