102676-47-1
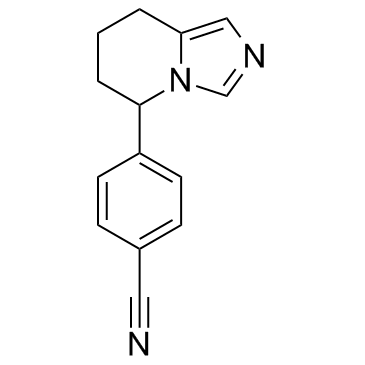
| Name | 4-(5,6,7,8-Tetrahydroimidazo[1,5-a]pyridin-5-yl)benzonitrile |
|---|---|
| Synonyms |
Fadrozole
UNII:H3988M64PU 5-p-cyanophenyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyridine 4-(5,6,7,8-Tetrahydroimidazo[1,5-a]pyridin-5-yl)benzonitrile Afema 4-(5,6,7,8-Tetrahydroimidazo1,5-apyridin-5-yl)benzonitrile 4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonitrile |
| Description | Fadrozole is a potent, selective and nonsteroidal inhibitor of aromatase with an IC50 of 6.4 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 6.4 nM (aromatase)[1] |
| In Vitro | Fadrozole hydrochloride is a very potent inhibitor of both human placental and rat ovarian aromatase. In hamster ovarian slices, fadrozole hydrochloride inhibits the production of estrogen with an IC50 of 0.03 μM. The production of progesterone is inhibited with an IC50 of 120 μM. Synthesis of other cytochrome P-450 dependent steroids can be suppressed to various degrees with higher doses of fadrozole hydrochloride. [1]. |
| In Vivo | Fadrozole hydrochloride is able to inhibit the aromatase-mediated androstenedione-induced uterine hypertrophy in immature female rats with an ED50 of 0.03 mg/kg when given orally. In the same model, aminoglutethimide elicits the same effect with an ED50 of 30 mg/kg when given orally[1]. Fadrozole hydrochloride prevents the development of both benign and malignant spontaneus mammary neoplasns in female Sprague-Dawley rats. It also slows the spontaneous development of ptuitary pars dta mas in female rats, and reduces the of spontaneous hcu ar tumours in male and female rats[2]. Administration of fadrozole in male and female mice suppresses the production of 17b-estradiol, accompanied with a 70% reduction in parasite burden. This protective effect is associated in male mice with a recovery of the specific cellular immune response. Interleukin-6 (IL-6) serum levels, and its production by splenocytes, is augmented by 80%, together with a 10-fold increase in its expression in testes of infected male mice. Fadrozole treatment returns these levels to baseline values[3]. |
| Animal Admin | Rats: Rats are treated with daily dosing with fadrozole hydrochloride (CGS 16949A) in purified water by gavage for 2 years. There are 60 rats in each of four groups given 0, 0.05, 0.25 or 1.25 mg/kg daily. Control rats receive only water. Clinical signs are recorded weekly and the animals are examine for palpable masses every 4 weeks for the first 9 months, then every 2 weeks for the remainder of the study[2]. Mice: Fadrozole is administered in the form of sub-dermal long-term release pellets (20 mg/wt kg, in three-week-release pellets), starting 1 week prior to the infection, using a 10-gauge needle. Three pellets are administrated during the study. Placebo pellets are administered to another group of infected mice, in the same fashion as the inhibitor. After 1 week, mice are infected and killed 8 weeks later[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.7±38.0 °C at 760 mmHg |
| Melting Point | 0ºC |
| Molecular Formula | C14H13N3 |
| Molecular Weight | 223.273 |
| Flash Point | 245.1±26.8 °C |
| Exact Mass | 223.110947 |
| PSA | 41.61000 |
| LogP | 1.88 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.662 |
| Storage condition | 2-8℃ |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
![6-cyano-2-[4-(ethoxycarbonyl)phenyl]pyridine structure](https://image.chemsrc.com/caspic/185/102676-39-1.png)
![4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-yl)benzoic acid structure](https://image.chemsrc.com/caspic/469/93178-73-5.png)