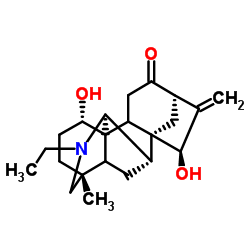
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WG0780000
-
CHEMICAL NAME :
-
Songorine
-
CAS REGISTRY NUMBER :
-
509-24-0
-
BEILSTEIN REFERENCE NO. :
-
0045107
-
LAST UPDATED :
-
199709
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C22-H31-N-O3
-
MOLECULAR WEIGHT :
-
357.54
-
WISWESSER LINE NOTATION :
-
T G5 D665 B6 B6/G-J/CL/LR A G- 5BBCCJ R CX FV HY JX MNTJ HU1 IQ M2 O1 RQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
408 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JOETD7 Journal of Ethnopharmacology. (Elsevier Scientific Pub. Ireland Ltd., POB 85, Limerick, Ireland) V.1- 1979- Volume(issue)/page/year: 4,247,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1575 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JOETD7 Journal of Ethnopharmacology. (Elsevier Scientific Pub. Ireland Ltd., POB 85, Limerick, Ireland) V.1- 1979- Volume(issue)/page/year: 4,247,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
485 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JOETD7 Journal of Ethnopharmacology. (Elsevier Scientific Pub. Ireland Ltd., POB 85, Limerick, Ireland) V.1- 1979- Volume(issue)/page/year: 4,247,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
630 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JOETD7 Journal of Ethnopharmacology. (Elsevier Scientific Pub. Ireland Ltd., POB 85, Limerick, Ireland) V.1- 1979- Volume(issue)/page/year: 4,247,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
25 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CTYAD8 Zhongcaoyao. Chinese Traditional and Herbal Medicine. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.11- 1980- Volume(issue)/page/year: 15,5,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
142 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85KYAH "Merck Index; an Encyclopedia of Chemicals, Drugs, and Biologicals", 11th ed., Rahway, NJ 07065, Merck & Co., Inc. 1989 Volume(issue)/page/year: 11,1374,1989
|