194423-15-9
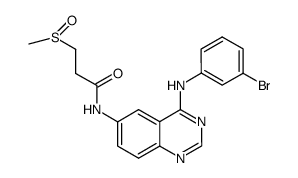
| Name | N-[4-(3-bromoanilino)quinazolin-6-yl]prop-2-enamide |
|---|---|
| Synonyms |
2-Propenamide (N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]
nchembio866-comp2 pd 168393 N-{4-[(3-Bromophenyl)amino]-6-quinazolinyl}acrylamide PD168393 |
| Description | PD168393 is an potent, cell-permeable, irreversible EGFR inhibitor with IC50 of 0.70 nM, irreversibly alkylate Cys-773, inactive against insulin, PDGFR, FGFR and PKC. target: EGFRIC 50: 0.7 nM [1](1) PD 168393 inhibite EGFr autophosphorylation in A431 human epidermoid carcinoma cells with >9-fold greater potency than PD 174265.[1](2) PD 168393 decrease the production of TNF-α and phosphrylation of ERK1/2 and p38 induced by LPS in cardiomyocytes.[2](3) PD168393 completely inhibits AKT and ERK phosphorylation at concentrations as low as 0.03 umol/L.[3](4) PD168393 could induce apoptosis and inhibit cell growth in ErbB2 positive lung and breast cancer cell lines.[3](5) PD168393 disrupted MEK1/p44/42 ERK signaling in HaCaT cells as determined by inhibition of phospho-p44/42 ERK. [4] |
|---|---|
| Related Catalog | |
| Target |
EGFR:0.7 nM (IC50) |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 571.1±50.0 °C at 760 mmHg |
| Melting Point | 279℃ |
| Molecular Formula | C17H13BrN4O |
| Molecular Weight | 369.215 |
| Flash Point | 299.2±30.1 °C |
| Exact Mass | 368.027252 |
| PSA | 70.40000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.744 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~43% 
194423-15-9 |
| Literature: Warner-Lambert Company Patent: US6344459 B1, 2002 ; Location in patent: Page column 48 ; US 6344459 B1 |
|
~84% 
194423-15-9 |
| Literature: Wyeth Patent: EP1100788 B1, 2005 ; Location in patent: Page/Page column 7 ; |
|
~56% 
194423-15-9 |
| Literature: Wyeth Patent: EP1100788 B1, 2005 ; Location in patent: Page/Page column 9-10 ; |
|
~49% 
194423-15-9 |
| Literature: Wyeth Patent: EP1100788 B1, 2005 ; Location in patent: Page/Page column 8 ; |
|
~11% 
194423-15-9 |
| Literature: Pawar, Vijaykumar G.; Sos, Martin L.; Rode, Haridas B.; Rabiller, Matthias; Heynck, Stefanie; Van Otterlo, Willem A. L.; Thomas, Roman K.; Rauh, Daniel Journal of Medicinal Chemistry, 2010 , vol. 53, # 7 p. 2892 - 2901 |
|
~% 
194423-15-9 |
| Literature: American Cyanamid Company Patent: EP787722 A1, 1997 ; Title/Abstract Full Text Show Details Wyeth Holdings Corporation Patent: EP980244 B1, 2003 ; |
|
~% 
194423-15-9 |
| Literature: Tsou; Mamuya; Johnson; Reich; Gruber; Ye; Nilakantan; Shen; Discafani; DeBlanc; Davis; Koehn; Greenberger; Wang; Wissner Journal of Medicinal Chemistry, 2001 , vol. 44, # 17 p. 2719 - 2734 |
|
~% 
194423-15-9 |
| Literature: Tsou; Mamuya; Johnson; Reich; Gruber; Ye; Nilakantan; Shen; Discafani; DeBlanc; Davis; Koehn; Greenberger; Wang; Wissner Journal of Medicinal Chemistry, 2001 , vol. 44, # 17 p. 2719 - 2734 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |