99009-20-8
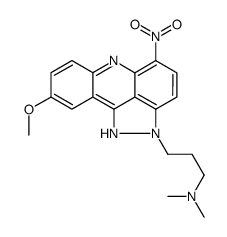
| Name | 3-(9-Methoxy-5-nitropyrazolo[3,4,5-kl]acridin-2(1H)-yl)-N,N-dimet hyl-1-propanamine |
|---|---|
| Synonyms |
Fluoren-9-yl-methyl-aether
9-Methoxy-fluoren 9-methoxyfluorene fluoren-9-yl-methyl ether 9-Methoxyellipticine 9-methoxy-9H-fluorene methoxyellipticine |
| Description | Pyrazoloacridine (NSC 366140), an intercalating agent with anti-cancer activity, inhibits the activity of topoisomerases 1 and 2. Pyrazoloacridine (NSC 366140) exhibits an IC50 of 1.25 μM in K562 myeloid leukemia cells for 24 h treatment[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Pyrazoloacridine (NSC 366140, PD 115934) exhibits IC50 values of 10.7 μM and 4.5 μM for oxic and hypoxic HCT-8 cells[1]. Pyrazoloacridine (NSC 366140, 2-4 μM) abolishes the catalytic activity of both topo I and topo II in vitro[2]. Pyrazoloacridine (NSC 366140) displays activity against cisplatin- and paclitaxel-resistant ovarian cancer[2]. Pyrazoloacridine (NSC 366140) has been shown to cause delayed DNA fragmentation in MCF-7 breast cancer cells[2]. Pyrazoloacridine (NSC 366140) induces apoptosis in P53-deficient Hep 3B human hepatoma cells[2]. Cell Cytotoxicity Assay[2] Cell Line: K562 Myeloid Leukemia Cells. Concentration: 0-500 μM. Incubation Time: 1 h or 24 h. Result: When K562 cells were incubated with PA for 1 h and then plated in soft agar, an IC50 of -50 μM was observed. In contrast, when cells were incubated for 24 h with PA, the IC50 was 1.25 μM. |
| References |
| Density | 1.314g/cm3 |
|---|---|
| Boiling Point | 595.6ºC at 760 mmHg |
| Molecular Formula | C19H21N5O3 |
| Molecular Weight | 367.40200 |
| Flash Point | 314ºC |
| Exact Mass | 367.16400 |
| PSA | 91.90000 |
| LogP | 4.06250 |
| Index of Refraction | 1.678 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|