1557268-88-8
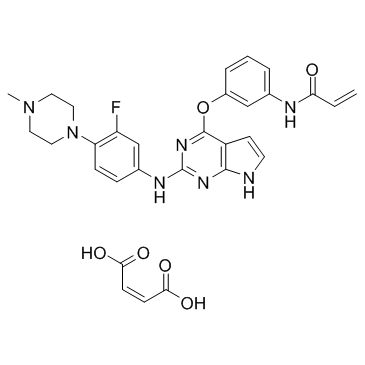
| Name | N-{3-[(2-{[3-Fluoro-4-(4-methyl-1-piperazinyl)phenyl]amino}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)oxy]phenyl}acrylamide (2Z)-2-butenedioate (1:1) |
|---|---|
| Synonyms |
N-{3-[(2-{[3-Fluoro-4-(4-methyl-1-piperazinyl)phenyl]amino}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)oxy]phenyl}acrylamide (2Z)-2-butenedioate (1:1)
2-Propenamide, N-[3-[[2-[[3-fluoro-4-(4-methyl-1-piperazinyl)phenyl]amino]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]phenyl]-, (2Z)-2-butenedioate (1:1) Avitinib maleate Avitinib (maleate) |
| Description | Avitinib maleate is a pyrrolopyrimidine-based irreversible epidermal growth factor receptor (EGFR) inhibitor with an IC50 of 7.68 nM. |
|---|---|
| Related Catalog | |
| Target |
EGFR:7.68 nM (IC50) |
| In Vitro | Avitinib is structurally distinct from previously reported pyrimidine-based irreversible EGFR inhibitors such as osimertinib and rociletinib. Avitinib is designed specifically to inhibit EGFR active mutations and the T790M acquired resistant mutation, while sparing wild type EGFR. Avitinib selectively inhibits EGFR active and T790M mutations with up to 298-fold increase in potency compared to wild-type EGFR. Avitinib exhibits potent inhibitory activity with IC50 value of 0.18 nM against EGFR L858R/T790M double mutations, nearly 43-fold greater potency over wild-type EGFR (IC50=7.68 nM). Avitinib selectively inhibits mutant EGFR phosphorylation with IC50 values of 7.3 nM and 2.8 nM in NCI-H1975 and NIH/3T3_TC32T8 cells, about 115- and 298-fold more sensitive than that of the inhibition of wild type EGFR in A431[1]. |
| In Vivo | Oral administration of avitinib at daily dose of 500 mg/kg results in complete remission of tumors with EGFR active and T790M mutations for over 143 days with no weight loss. Three major metabolites of avitinib are tested and show no wild-type EGFR inhibition and off-target effects such as inhibition of IGF-1R. Avitinib is safe in non-small cell lung cancer (NSCLC) patients at the dose range between 50 mg and 550 mg once per day and no hyperglycemia and other severe adverse effects are detected such as grade 3 QT prolongation[1]. |
| Cell Assay | Cell proliferation is assayed by a cell viability reagent, WST-1. Cells are seeded at optimal density onto 96-well plates and incubated for 24 hours, followed by avitinib treatment for 72 hours. Cell viability is then assayed by incubating cells with WST-1 reagent for 2-3 hrs[1]. |
| Animal Admin | Rats: To assess the potential skin toxicity of avitinib, a rat model is used. Rats are administrated daily with avitinib at 300 mg/kg for 4 weeks, and in control groups gefitinib at 50mg/kg or vehicle control (0.5% MC) is administrated[1]. Mice: NCI-H1975 tumor bearing mice are orally treated with a vehicle control (0.5% MC), avitinib at dose levels of 12.5 mg/kg and 50 mg/kg for 17 days when TV value in vehicle control group reached approximately 2000 mm3 . After 17-day dosing, animals in the vehicle control group are sacrificed, whereas animals in avitinib groups are continually daily administrated with increased dose at 500 mg/kg till the test mice cannot tolerate the treatment. Mouse body weight and TV are measured twice per week. TV is then used for the calculation of tumor inhibitory rate and tumor regression rate[1]. |
| References |
| Molecular Formula | C30H30FN7O6 |
|---|---|
| Molecular Weight | 603.601 |
| Exact Mass | 603.224182 |
| Storage condition | -20℃ |