851528-79-5
| Name | seladelpar |
|---|---|
| Synonyms |
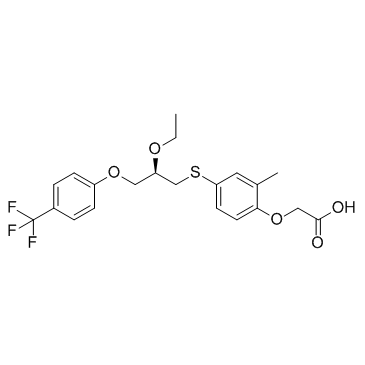
[4-({(2R)-2-Ethoxy-3-[4-(trifluoromethyl)phenoxy]propyl}sulfanyl)-2-methylphenoxy]acetic acid
UNII:7C00L34NB9 |
| Description | Seladelpar is an orally active, potent (50% effect concentration EC50 2 nM), and specific PPAR-δ agonist. |
|---|---|
| Related Catalog | |
| Target |
PPAR-δ:2 nM (EC50) PPAR-α:1600 nM (EC50) |
| In Vitro | Seladelpar (MBX-8025) is an orally active, potent (2 nM), and specific (>750-fold and >2500-fold compared with PPAR-α or PPAR-γ receptors, respectively) PPAR-δ agonist being developed as a lipid-altering agent[1]. Seladelpar is a potent, and selective PPAR-δ agonist (50% effect concentration human PPAR-δ=2 nM, PPAR-α=1,600 nM) that demonstrates favorable effects on insulin resistance, diabetes, and atherogenic dyslipidemia[2]. |
| In Vivo | From weaning, female Alms1 mutant (foz/foz) mice and wild-type littermates are fed an atherogenic diet for 16 weeks; groups (n=8-12) are then randomized to receive Seladelpar (10 mg/kg) or vehicle (1% methylcellulose) by gavage for 8 weeks. Despite minimally altering body weight, Seladelpar normalizes hyperglycemia, hyperinsulinemia, and glucose disposal in foz/foz mice. Serum alanine aminotransferase ranges 300-600 U/L in vehicle-treated foz/foz mice; Seladelpar reduces alanine aminotransferase by 50%. In addition, Seladelpar normalizes serum lipids and hepatic levels of free cholesterol and other lipotoxic lipids that are increased in vehicle-treated foz/foz versus wild-type mice. This abolished hepatocyte ballooning and apoptosis, substantially reduce steatosis and liver inflammation, and improve liver fibrosis. In vehicle-treated foz/foz mice, the mean nonalcoholic fatty liver disease activity score is 6.9, indicating nonalcoholic steatohepatitis (NASH); Seladelpar reverses NASH in all foz/foz mice (nonalcoholic fatty liver disease activity score 3.13). In atherogenic diet-fed Wt mice, administration of Seladelpar reduces body weight by ∼18% (P<0.05). In contrast, Seladelpar produces minimal effect on body weight in atherogenic diet–fed foz/foz mice. These animals develope severe hyperglycemia, hyperinsulinemia, and whole-body insulin resistance after 16 weeks (P<0.05); Seladelpar strikingly improves these indices (P<0.05). After intraperitoneal glucose injection, blood glucose reaches ~32 mM in vehicle-treated versus ~14 mM in Seladelpar-treated foz/foz mice (P<0.05); the area under the blood glucose disappearance curve is correspondingly lower in Seladelpar-treated foz/foz mice (P<0.05). Seladelpar produces a proportionally similar effect on glucose handling in atherogenic diet–fed Wt mice (P<0.05)[2]. |
| Animal Admin | Mice[2] From weaning (week 4), Alms1 mutant (foz/foz) NOD.B10 mice or Wt littermates (female mice in both groups) are fed an atherogenic diet (23% fat, 0.2% cholesterol and 45% simple carbohydrate; 4.78 kcal/g digestible energy) ad libitum for 16 weeks, after which groups are randomized (n=8-12 mice/group) to once-a-day oral administration (by gavage) for 8 weeks of Seladelpar (10 mg/kg in 1% methylcellulose) or vehicle (controls). Animals are housed under 12-hour light/dark cycle and constant temperature of 22°C and receive maximal humane care[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 557.5±50.0 °C at 760 mmHg |
| Molecular Formula | C21H23F3O5S |
| Molecular Weight | 444.465 |
| Flash Point | 291.0±30.1 °C |
| Exact Mass | 444.121826 |
| LogP | 6.16 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.554 |
| Storage condition | -20℃ |