1155419-89-8
| Name | BI 224436 |
|---|---|
| Synonyms |
99A996378Y
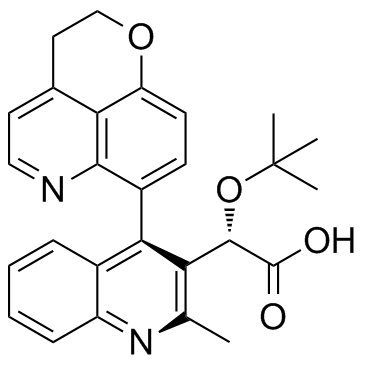
BI 224436 (2S)-[4-(2,3-Dihydropyrano[4,3,2-de]quinolin-7-yl)-2-methyl-3-quinolinyl][(2-methyl-2-propanyl)oxy]acetic acid |
| Description | BI 224436 is a novel HIV-1 noncatalytic site integrase inhibitor with EC50 values of less than 15 nM against different HIV-1 laboratory strains. |
|---|---|
| Related Catalog | |
| Target |
EC50: 15 nM (HIV-1)[1] |
| In Vitro | BI 224436 has cellular cytotoxicity of more than 90 μM. BI 224436 has a low, 2.1-fold decrease in antiviral potency in the presence of 50% human serum. BI 224436 retains full antiviral activity against recombinant viruses encoding INSTI resistance substitutions N155S, Q148H, and E92Q. BI 224436 displays an additive effect in combination with most approved antiretrovirals, including INSTIs. BI 224436 has drug-like in vitro absorption, distribution, metabolism, and excretion (ADME) properties, including Caco-2 cell permeability, solubility, and low cytochrome P450 inhibition[1]. |
| In Vivo | BI 224436 exhibits excellent pharmacokinetic profiles in rat (clearance as a percentage of hepatic flow [CL], 0.7%; bioavailability [F], 54%), monkey (CL, 23%; F, 82%), and dog (CL, 8%; F, 81%)[1]. |
| Kinase Assay | BI 224436 is dissolved in acetonitrile-methanol (50:50, vol/vol) to achieve a concentration of 1.5 mM. Phosphate buffer (pH 7.4), cofactor, and test substance or isoform-selective inhibitors are added to 96-well plates and are prewarmed to 37°C for 10 min. Cofactor concentrations are 1.3 mM NADP, 3.3 mM glucose-6-phosphate, and 0.4 U/mL glucose-6-phosphate dehydrogenase. Reactions are initiated by the addition of prewarmed (37°C) enzyme and substrate. Reaction mixtures are incubated at 37°C and terminated by the addition of 0.038 ml of 40:40:20 (vol/vol) methanol–acetonitrile–0.5 M Tris buffer. Formation of the fluorescent metabolites is measured using a microplate spectrofluorometer at specific excitation and emission wavelengths. The IC50 is determined using the 96-well 32 procedure supplied with the SAS software[1]. |
| Animal Admin | Rats: For oral PK studies, BI 224436 is administered in a suspension of 0.5% (wt/vol) methyl cellulose (MC), 0.3% (vol/vol) Tween 80, and 1% (vol/vol) N-methyl-2-pyrrolidine (MP) in water. For i.v. dosing, BI 224436 is dissolved in 70% PEG 400–30% water (vol/vol). The appropriate amount of BI 224436 is dissolved in PEG 400 with sonication. The rats receive a single i.v. dose of 0.2 mg/kg of body weight (1 mL/kg) via the jugular vein as a bolus or received a single oral dose of 0.4 mg/kg (10 mL/kg) administered by gavage. Blood samples are obtained at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 32 h after dosing for analysis[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 631.8±55.0 °C at 760 mmHg |
| Molecular Formula | C27H26N2O4 |
| Molecular Weight | 442.506 |
| Flash Point | 335.9±31.5 °C |
| Exact Mass | 442.189270 |
| LogP | 4.11 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Storage condition | 2-8℃ |