1254036-71-9
| Name | GSK2269557 free base |
|---|---|
| Synonyms |
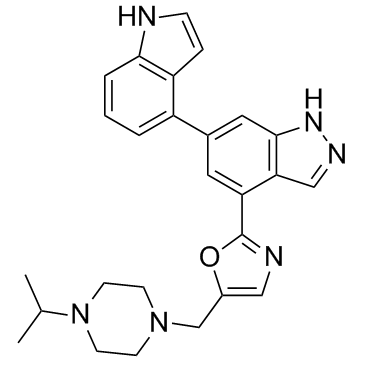
6-(1H-Indol-4-yl)-4-{5-[(4-isopropyl-1-piperazinyl)methyl]-1,3-oxazol-2-yl}-1H-indazole
UNII:OEP8JJ3OZR OEP8JJ3OZR Nemiralisib GSK2269557 (free base) |
| Description | GSK2269557 free base is a potent and highly selective PI3Kδ inhibitor with a pKi of 9.9. |
|---|---|
| Related Catalog | |
| Target |
PI3Kδ:9.9 (pKi) PI3Kγ:5.2 (pIC50) PI3Kα:5.3 (pIC50) PI3Kβ:5.8 (pIC50) |
| In Vitro | GSK2269557 free base is highly selective for PI3Kδ, with >1000-fold selectivity over the closely related isoforms PI3Kα (pIC50=5.3), PI3Kβ (pIC50=5.8) and PI3Kγ (pIC50=5.2). GSK2269557 free base inhibits IFNγ in the peripheral blood mononuclear (PBMC) assay with an pIC50 of 9.7[1]. |
| In Vivo | To assess the suitability of the series for inhaled delivery clearance data in rat microsomes and subsequently in vivo pharmacokinetic data from Sprague Dawley male rats is obtained. Compounds (e.g., GSK2269557) are administered by the oral or intravenous routes, at a dose level of 3 and 1mg/kg respectively (n=2 rats/route). GSK2269557 free base is active in a disease relevant brown norway rat acute OVA model of Type 2 helper T-cells (Th2)-driven lung inflammation[1]. |
| Kinase Assay | Inhibition of PI3Kinase enzymatic activity is determined using a homogeneous time resolved fluorescence (HTRF) kit assay format. Reactions are performed in assay buffer containing 50 mM HEPES, pH 7.0, 150 mM NaCl, 10 mM MgCl2, <1 % cholate (w/v), <1 % CHAPS (w/v), 0.05 % sodium azide (w/v) and 1 mM DTT. Enzymes are preincubated with compound, serially diluted 4-fold in 100 % DMSO, for 15 mins prior to reaction initiation upon addition of substrate solution containing ATP at Km for the specific isoform tested (PI3Kα at 250 μM, PI3Kβ at 400 μM, PI3Kδ at 80 μM and PI3Kγ at 15 μM), PIP2 at either 5 μM (PI3Kδ) or 8 μM (PI3Kα, PI3Kβ and PI3Kγ) and 10 nM biotin-PIP3. Assays are quenched after 60 mins by addition of a quench/detection solution prepared in 50 mM HEPES pH 7.0, 150 mM NaCl, <1 % cholate, <1 % Tween 20, 30 mM EDTA, 40 mM potassium fluoride and 1 mM DTT containing 16.5 nM GRP-1 PH domain, 8.3 nM Streptavidin-APC and 2 nM Europium-anti-GST, and are left for a further 60 mins in the dark to equilibrate prior to reading using a BMG RubyStar plate reader. Ratio data are normalised to high (no compound) and low (no enzyme) controls prior to fitting using a logistical four parameter equation to determine IC50[1]. |
| Animal Admin | Rats[1] In vivo pharmacokinetics is tested in Sprague Dawley male rats. Compounds (e.g., GSK2269557) are administered discretely by the oral or intravenous routes, at a dose level of 3 and 1 mg/kg respectively (n=2 rats/route). Compounds (e.g., GSK2269557) are formulated as a solution in DMSO:PEG200:water (5:45:50 v/v/v) at a dose volume of 6 (oral) and 2 (intravenous) mL/kg. All animals are serially bled from the tail vein and blood samples collected over a time-course of 0-7 h are submitted to LC-MS/MS analysis for the quantification of the parent compound. The main pharmacokinetic parameters are estimated by non-compartmental analysis. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 706.2±70.0 °C at 760 mmHg |
| Molecular Formula | C26H28N6O |
| Molecular Weight | 440.540 |
| Flash Point | 380.9±35.7 °C |
| Exact Mass | 440.232452 |
| LogP | 3.23 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.686 |
| Storage condition | -20℃ |