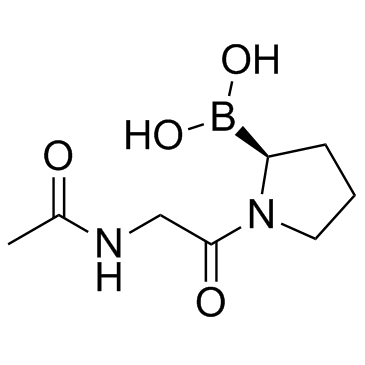
886992-99-0
| Name | Ac-Gly-BoroPro |
|---|---|
| Synonyms | [(2S)-1-(N-Acetylglycyl)-2-pyrrolidinyl]boronic acid |
| Description | Ac-Gly-BoroPro is a selective FAP inhibitor with a Ki of 23 nM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 23 nM (FAP)[1] |
| In Vitro | FAP has been implicated in cancer; however, its specific role remains elusive because inhibitors that distinguish FAP from other prolyl peptidases like dipeptidyl peptidase-4 (DPP-4) have not been developed. Ac-Gly-BoroPro selectively inhibits FAP relative to other prolyl peptidases. FAP reacts readily with submicromolar concentrations of Ac-Gly-BoroPro, reaching steady state inhibition levels rapidly (Ki=23±3 nM). In contrast, DPP-4 requires higher Ac-Gly-BoroPro concentrations for inhibition and a longer time to reach steady state inhibition levels (Ki=377±18 nM). Ac-Gly-BoroPro inhibits other prolyl peptidases (DPP-7, DPP-8, DPP-9, prolyl oligopeptidase, and acylpeptide hydrolase) with Ki values ranging from 9- to 5400-fold higher than that for FAP inhibition. The N-acyl-linkage in Ac-Gly-BoroPro blocks the N terminus of the inhibitor, making it less nucleophilic and therefore unlikely to cyclize[1]. |
| Kinase Assay | Ki values for inhibition of proteases by Ac-Gly-BoroPro are determined using the method of progress curves for analysis of tight binding competitive inhibitors. Various concentrations of Ac-Gly-BoroPro are reacted with FAP (1.0 nM) and DPP-4 (0.1 nM) in the presence of Ala-Pro-AFC (500 μM for FAP; 100 μM for DPP-4), and time-dependent inhibition of each protease is monitored. Reactions contained inhibitor concentrations at least 20-fold greater than protease concentrations, such that the protease-inhibitor complex does not significantly deplete the free inhibitor[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C8H15BN2O4 |
| Molecular Weight | 214.027 |
| Exact Mass | 214.112488 |
| LogP | -1.21 |
| Index of Refraction | 1.520 |
| Storage condition | -20°C |