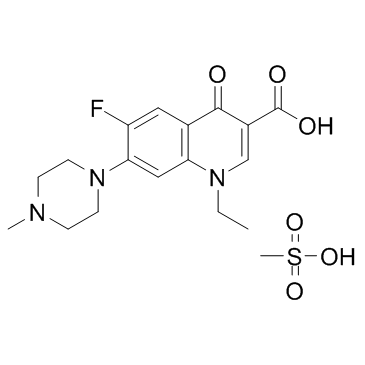
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VB2002200
-
CHEMICAL NAME :
-
3-Quinolinecarboxylic acid, 1,4-dihydro-1-ethyl-6-fluoro-7-(4-methyl-1-piperaziny l)-4-oxo-, monomethanesulfonate, dihydrate
-
CAS REGISTRY NUMBER :
-
70458-95-6
-
LAST UPDATED :
-
199009
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C17-H20-F-N3-O3.C-H4-O3-S.2H2-O
-
MOLECULAR WEIGHT :
-
465.55
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CHDDAT Comptes Rendus des Seances de l'Academie des Sciences, Serie D: Sciences Naturelles. (Paris, France) V.262-291, 1966-80. For publisher information, see CRASEV. Volume(issue)/page/year: 292,37,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CHDDAT Comptes Rendus des Seances de l'Academie des Sciences, Serie D: Sciences Naturelles. (Paris, France) V.262-291, 1966-80. For publisher information, see CRASEV. Volume(issue)/page/year: 292,37,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CHDDAT Comptes Rendus des Seances de l'Academie des Sciences, Serie D: Sciences Naturelles. (Paris, France) V.262-291, 1966-80. For publisher information, see CRASEV. Volume(issue)/page/year: 292,37,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
225 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CHDDAT Comptes Rendus des Seances de l'Academie des Sciences, Serie D: Sciences Naturelles. (Paris, France) V.262-291, 1966-80. For publisher information, see CRASEV. Volume(issue)/page/year: 292,37,1981
|