120693-53-0
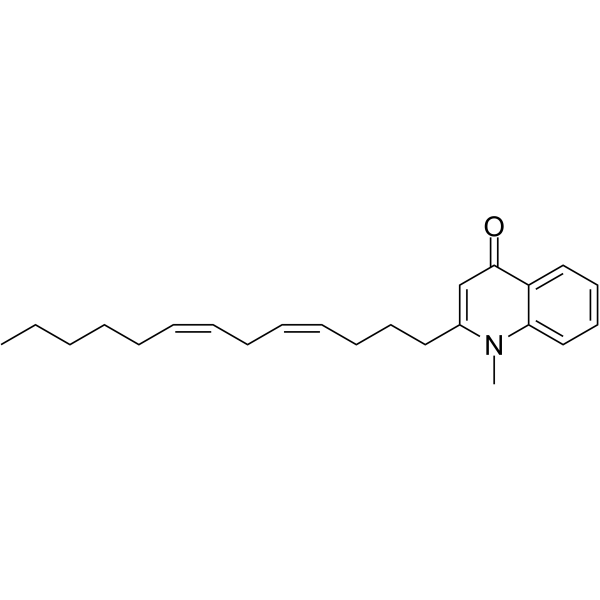
| Name | 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone |
|---|
| Description | 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone, a quinolone alkaloid, is a diacylglycerol acyltransferase inhibitor and angiotensin II receptor blocker, with IC50s of 20.1 μM and 34.1 μM, respectively. 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone shows potent anti-Helicobacter pylori activity with the MIC of 10 μg/mL[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 20.1 μM (diacylglycerol acyltransferase)[3], 34.1 μM (angiotensin II receptor)[2] |
| In Vitro | 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone shows anti-Helicobacter pylori activity, with the MIC of 10 μg/mL, and has no effect on Helicobacter pylori urease activity at the concentration of 300 μg/mL[1]. 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (5-500 μM) shows a dose-dependent diacylglycerol acyltransferase (DGAT) inhibition, with an IC50 of 20.1 μM[3]. 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (5-20 μM) inhibits leukotriene biosynthesis in human polymorphonuclear granulocytes, with an IC50 of 10.1 μM[4]. |
| References |
| Molecular Formula | C23H31NO |
|---|---|
| Molecular Weight | 337.50 |