1299440-37-1
| Name | Inebilizumab |
|---|
| Description | Inebilizumab is an anti-CD19 monoclonal antibody (mAb) with enhanced antibody-dependent cell-mediated cytotoxicity against B cells. Inebilizumab can be used for multiple sclerosis and neuromyelitis optica research[1]. |
|---|---|
| Related Catalog | |
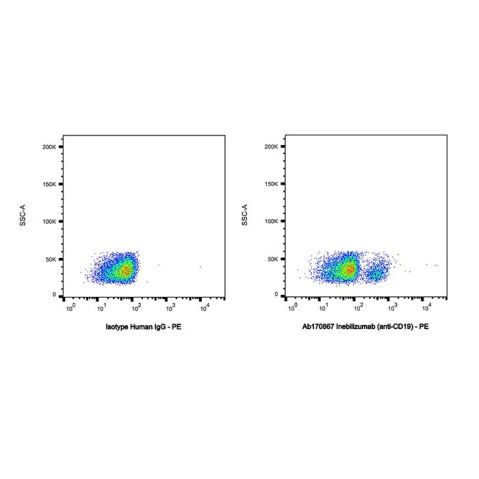
| In Vitro | Inebilizumab is derived from the mouse anti-human mAb HB12b, which had already shown impressive activity in depletion of B cells in transgenic mice carrying the human CD19 gene (hCD19 Tg)[1]. Inebilizumab potently depletes CD19-expressing B cells, including primary human B cells, B cell lines derived from multiple tumor types, and neoplastic B cells[1]. Inebilizumab demonstrates equal or better activity than Rituximab (HY-P9913) in depletion of human primary B cells in autologous ADCC assays and shows potent ADCC activity against human in vitro-differentiated and primary plasma cells[1]. |
| In Vivo | Inebilizumab (MEDI-551) (0-10 mg/kg; i.v.; once) depletes B cells from blood and spleen by mouse macrophages in vivo and phagocytosis of murine B cells ex vivo[2]. Animal Model: huCD19/CD20 double Tg mice[2] Dosage: 0.5, 2, or 10 mg/kg Administration: Tail vein injection, once Result: Depleted B cells from blood and spleen, B-cell depletion in blood and spleen was maintained for more than 2 weeks after a single 10 mg/kg administration (better than Rituximab). Resulted in a substantial reduction (on average by 91.4% by day 3) in BM B220+muCD19+ B cells. Led to depletion of B cell by mouse macrophages. |
| References |
| No Any Chemical & Physical Properties |