6640-90-0
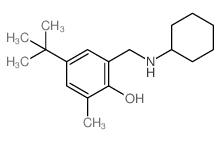
| Name | 4-tert-butyl-2-[(cyclohexylamino)methyl]-6-methylphenol |
|---|---|
| Synonyms | 4-tert-Butyl-2-cyclohexylaminomethyl-6-methyl-phenol |
| Description | NSC 48160 inhibits the growth of the pancreatic cancer cells with IC50s of 84.3 μM for CPFAC-1 and 94.5 μM for BxPC-3. NSC 48160 also induces pancreatic cancer cell apoptosis. NSC 48160 can improve metabolic syndromes, such as NASH, obesity and lipid metabolism disorders[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1g/cm3 |
|---|---|
| Boiling Point | 387.3ºC at 760 mmHg |
| Molecular Formula | C18H29NO |
| Molecular Weight | 275.42900 |
| Flash Point | 67.2ºC |
| Exact Mass | 275.22500 |
| PSA | 32.26000 |
| LogP | 4.81130 |
| Index of Refraction | 1.538 |
|
~% 
6640-90-0 |
| Literature: Burke et al. Journal of the American Chemical Society, 1958 , vol. 80, p. 3438,3443 |
|
~% 
6640-90-0 |
| Literature: Burke et al. Journal of the American Chemical Society, 1958 , vol. 80, p. 3438,3443 |
|
~% 
6640-90-0 |
| Literature: Burke et al. Journal of the American Chemical Society, 1958 , vol. 80, p. 3438,3443 |