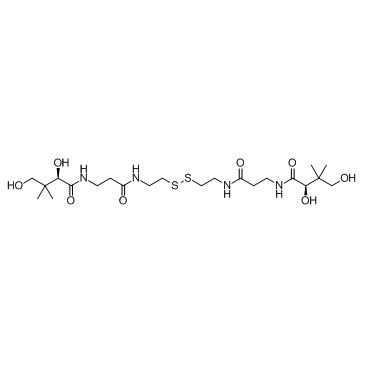
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
ES4392700
-
CHEMICAL NAME :
-
Butyramide, N,N'-(dithiobis(ethyleneiminocarbonylethylene))bis(2, 4-dihydroxy-3,3-dimet hyl-, D-(+)-
-
CAS REGISTRY NUMBER :
-
16816-67-4
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C22-H42-N4-O8-S2
-
MOLECULAR WEIGHT :
-
554.80
-
WISWESSER LINE NOTATION :
-
Q1X1&1&YQVM2VM2MV2MVYQX1&1&1Q
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IQAH "Modern Pharmaceuticals of Japan, IV," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1972 Volume(issue)/page/year: -,98,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4810 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IQAH "Modern Pharmaceuticals of Japan, IV," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1972 Volume(issue)/page/year: -,98,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>4870 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IQAH "Modern Pharmaceuticals of Japan, IV," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1972 Volume(issue)/page/year: -,98,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3410 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IQAH "Modern Pharmaceuticals of Japan, IV," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1972 Volume(issue)/page/year: -,98,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IQAH "Modern Pharmaceuticals of Japan, IV," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1972 Volume(issue)/page/year: -,98,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,598,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,598,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4870 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,598,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,598,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,598,1982 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1344 gm/kg/24W
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Nutritional and Gross Metabolic - other changes Related to Chronic Data - death
-
REFERENCE :
-
85IOAB "Modern Pharmaceuticals of Japan, VII," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Association, 1985 Volume(issue)/page/year: -,73,1985 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 2,55,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 2,55,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
9 gm/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 2,55,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 2,55,1968
|