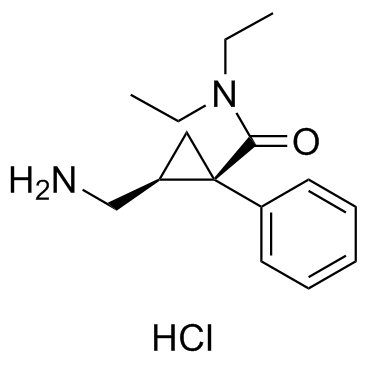
101152-94-7
| Name | Milnacipran Hydrochloride |
|---|---|
| Synonyms |
2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride (1:1)
EINECS 200-659-6 1-Phenyl-1-(diethylaminocarbonyl)-2-(aminomethyl)cyclopropane hydrochloride Cyclopropanecarboxamide, 2-(aminomethyl)-N,N-diethyl-1-phenyl-, hydrochloride (1:1) (1R,2S)-rel-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide monohydrochloride Midalcipran MFCD00901293 Midalcipran hydrochloride cis-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide Hydrochloride (1R,2S)-rel-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride Cyclopropanecarboxamide, 2-(aminomethyl)-N,N-diethyl-1-phenyl-, (1R,2S)-, hydrochloride (1:1) Milnacipran hydrochloride (1R,2S)-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride (1:1) UNII:RNZ43O5WW5 (1R-2S)-Milnacipran hydrochloride Milnacipran (hydrochloride) |
| Description | Milnacipran hydrochloride is a serotonin-norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia.Target: SNRIMilnacipran (Ixel, Savella, Dalcipran, Toledomin) is a serotonin-norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia. It is not approved for the clinical treatment of major depressive disorder in the USA, but it is in other countries.Milnacipran inhibits the reuptake of serotonin and norepinephrine in an approximately 1:3 ratio, respectively; in practical use this means a relatively balanced action upon bothneurotransmitters. Increasing both neurotransmitters concentration simultaneously works synergistically to treat both depression and fibromyalgia. Milnacipran exerts no significant actions onH1, α1, D1, D2, and mACh receptors, as well as on benzodiazepine and opioid binding sites. Milnacipran is well absorbed after oral dosing and has a bioavailability of 85%. Meals do not have an influence on the rapidity and extent of absorption. Peak plasma concentrations are reached 2 hours after oral dosing. The elimination half-life of 8 hours is not increased by liver impairment and old age, but by significant renal disease. Milnacipran is conjugated to the inactive glucuronide and excreted in the urine as unchanged drug and conjugate. Only traces of active metabolites are found. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 393ºC at 760 mmHg |
|---|---|
| Melting Point | 179-181ºC |
| Molecular Formula | C15H23ClN2O |
| Molecular Weight | 282.809 |
| Exact Mass | 282.149902 |
| PSA | 46.33000 |
| LogP | 3.27370 |
| Vapour Pressure | 1.66E-07mmHg at 25°C |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 19 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 7-16-36/37-45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | GZ1014010 |
| Flash Point(F) | 48.2 °F |
| Flash Point(C) | 9 °C |