7177-50-6
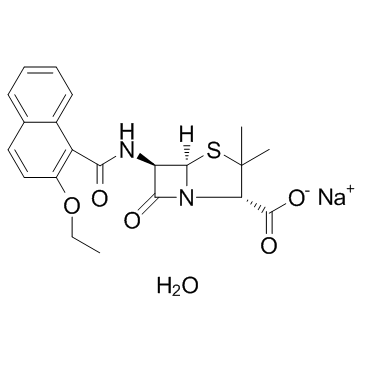
| Name | nafcillin sodium monohydrate |
|---|---|
| Synonyms |
nafcillin sodium monohydrate
MFCD01941128 Nafcillin sodium salt monohydrate NAFCILLINSODIUMSTERILE nafcillin sodium Unipen (tn) Sodium (2S,5R,6R)-6-[(2-ethoxy-1-naphthoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate hydrate (1:1:1) Nafcillin monohydrate sodium salt 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2-ethoxy-1-naphthalenyl)carbonyl]amino]-3,3-dimethyl-7-oxo-, sodium salt, (2S,5R,6R)-, hydrate (1:1:1) Nafcillin (sodium monohydrate) |
| Description | Nafcillin sodium monohydrate is a semi-synthetic antibiotic related to penicillin.Target: AntibacterialNafcillin sodium is a narrow-spectrum, beta-lactam antibiotic of the penicillin class. As a beta-lactamase-resistant penicillin, it is used to treat infections caused by Gram-positive bacteria, in particular, species of staphylococci that are resistant to other penicillins. Nafcillin is considered therapeutically equivalent to oxacillin, although its safety profile is somewhat different. Nafcillin was shown to reversibly inhibit beta-lactamase from Staphylococcus aureus PC1 with characteristics indicative of a type A inhibitor [Citri, Samuni & Zyk (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 1048-1052]. At nafcillin concentrations above 80 mM, complete inactivation occurred within 200 s. Upon removal of the excess nafcillin the inhibited enzyme was re-activated completely, with a rate constant of 2.0 x 10(-3) s-1 (25 degrees C) [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.42 g/cm3 |
|---|---|
| Boiling Point | 714.1ºC at 760 mmHg |
| Molecular Formula | C21H23N2NaO6S |
| Molecular Weight | 454.472 |
| Flash Point | 385.7ºC |
| Exact Mass | 454.117462 |
| PSA | 133.30000 |
| LogP | 1.41360 |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |