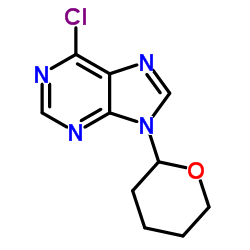
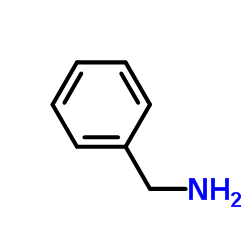
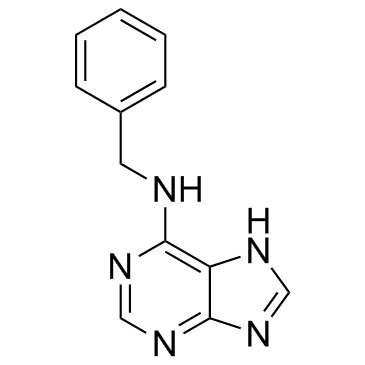
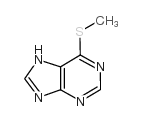
2312-73-4
| Name | N-Benzyl-9-(2-tetrahydropyranyl)adenine |
|---|---|
| Synonyms |
Pyranyl benzyladenine
MFCD00056971 N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)-9H-purin-6-amine UNII:23NQ6S177V N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine 6-Benzylamino-9-(2-tetrahydropyranyl)-9H-purine,BPA |
| Description | BAP9THP is a synthetic cytokinin derivative and a growth regulator. BAP9THP promotes chlorophyll retention (and senescence delay) in plant tissues exceptionally strongly, and growth of tobacco callus almost as strongly as 6-Benzylaminopurine (BAP). BAP9THP induces adventitious shoot formation ignificantly more strongly than N6-isopentenyladenine or Kinetin[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | The cytokinin derivative BAP9THP is an important component of these protocols: culture of isolated apical meristem or growing shoot apices on media containing this compound resulted in signifcant shoot multiplication[1]. both BAP9THP and BAP9THF are found to delay senescence and induce several growth responses more strongly than BAP[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.1±60.0 °C at 760 mmHg |
| Melting Point | 110-114ºC |
| Molecular Formula | C17H19N5O |
| Molecular Weight | 309.366 |
| Flash Point | 281.1±32.9 °C |
| Exact Mass | 309.158966 |
| PSA | 64.86000 |
| LogP | 2.45 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.700 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn:Harmful; |
|---|---|
| Risk Phrases | R22 |
| Safety Phrases | S26-S36 |
| WGK Germany | 3 |
| RTECS | UO7510000 |
|
~23% 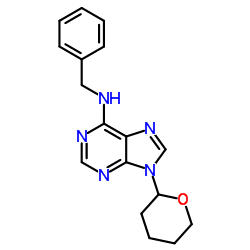
2312-73-4 |
| Literature: Gasque, C. Edward Phytochemistry (Elsevier), 1982 , vol. 21, # 7 p. 1501 - 1508 |
|
~71% 
2312-73-4 |
| Literature: Villar, Jose Daniel Figueroa; Motta, Marita Almeida Nucleosides, Nucleotides and Nucleic Acids, 2000 , vol. 19, # 5-6 p. 1005 - 1015 |
|
~% 
2312-73-4 |
| Literature: Nucleosides, Nucleotides and Nucleic Acids, , vol. 19, # 5-6 p. 1005 - 1015 |
|
~% 
2312-73-4 |
| Literature: Nucleosides, Nucleotides and Nucleic Acids, , vol. 19, # 5-6 p. 1005 - 1015 |
|
~% 
2312-73-4 |
| Literature: Nucleosides, Nucleotides and Nucleic Acids, , vol. 19, # 5-6 p. 1005 - 1015 |