120788-07-0
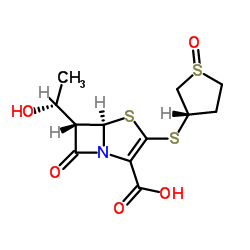
| Name | Sulopenem |
|---|---|
| Synonyms |
(5R,6S)-6-[(1R)-1-Hydroxyethyl]-7-oxo-3-[[(3S)-tetrahydro-3-thienyl]thio]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (R)-S-oxide
(3S)-3-({(5R (5R,6S)-6-(1(R)-Hydroxyethyl)-2-[(1(R)-oxo-3(S)-thiolanyl)thio]-2-penem-3-carboxylic acid (5R,6S)-6-[(1R)-1-Hydroxyethyl]-3-{[(3S)-1-oxidotetrahydro-3-thiophenyl]sulfanyl}-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid CP-70,429 (5R,6S)-6-[(1R)-1-Hydroxyethyl]-3-{[(3S)-1-oxidotetrahydrothiophen-3-yl]sulfanyl}-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid 6S)-2-carboxy-6-[(1R)-1-hydroxyethyl]-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-en-3-yl}sulfanyl)tetrahydrothiophenium-1-olate |
| Description | Sulopenem (CP-70429) is an orally active, parenteral penem antibiotic with broad-spectrum activities against Gram-positive and Gram-negative bacteria. Sulopenem has the potential for urinary tract infections and intra-abdominal infections treatment. Sulopenem is inactive against Pseudomonas aeruginosa and Xanthomonas maltophilia[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
MIC: 0.015-0.12 µg/mL (E. coli)[1] |
| In Vitro | Sulopenem has the potential for uncomplicated and complicated urinary tract infections and intra-abdominal infections treatment, including multidrug-resistant (MDR) infections and infections attributable to quinolone-nonsusceptible and/or extended-spectrum β-lactamase (ESBL)-producing Gram-negative bacilli[1]. Sulopenem inhibits the growth of most isolates of aerobic and anaerobic Gram-positive and Gram-negative bacteria, including methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (penicillin-susceptible and -resistant isolates), group A and B β-hemolytic streptococci, Listeria monocytogenes, Enterobacteriaceae, Haemophilus influenzae, and Moraxella catarrhalis but excluding P. aeruginosa and Stenotrophomonas maltophilia, at a concentration of ≤1 μg/mL[1]. |
| In Vivo | The protective effects of Sulopenem in murine experimental systemic infections are superior to those of Imipenem/Cilastatin. In murine experimental mixed infection with Escherichia coli and Bacteroides fragilis, Sulopenem has lower ED50. In guinea pigs experimental lung infection with Klebsiella pneumoniae, Sulopenem is more effective than CZON or Cefotiam[3]. |
| References |
| Density | 1.74g/cm3 |
|---|---|
| Boiling Point | 693.1ºC at 760mmHg |
| Molecular Formula | C12H15NO5S3 |
| Molecular Weight | 349.44600 |
| Flash Point | 373ºC |
| Exact Mass | 349.01100 |
| PSA | 164.72000 |
| LogP | 1.21000 |
| Vapour Pressure | 3.2E-22mmHg at 25°C |
| Index of Refraction | 1.768 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |