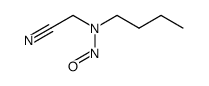
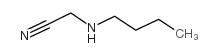
3483-14-5
| Name | 5-amino-3-bromoindazole |
|---|---|
| Synonyms |
3-butylsydnonimine hydrochorid
3-bromo-1H-indazole-5-ylamine 3-bromo-1H-indazol-5-ylamine 3-bromo-1H-indazol-5-amine 3-butylsydnonimine hydrochloride 1H-Indazol-5-amine,3-bromo |
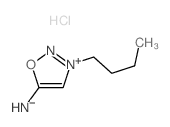
| Molecular Formula | C6H12ClN3O |
|---|---|
| Molecular Weight | 177.63200 |
| Exact Mass | 177.06700 |
| PSA | 41.94000 |
| LogP | 2.24790 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
3483-14-5 |
| Literature: Beal; Turnbull Synthetic Communications, 1992 , vol. 22, # 5 p. 673 - 676 |
|
~% 
3483-14-5 |
| Literature: Beal; Turnbull Synthetic Communications, 1992 , vol. 22, # 5 p. 673 - 676 |