1404-93-9
| Name | Vancomycin Hydrochloride |
|---|---|
| Synonyms |
(1S,2R,18R,19R,22S,25R,28R,40S)-22-(2-Amino-2-oxoethyl)-48-{[2-O-(3-amino-2,3,6-trideoxy-3-methyl-α-L-lyxo-hexopyranosyl)-β-D-glucopyranosyl]oxy}-5,15-dichloro-2,18,32,35,37-pentahydroxy-19-[(N -methyl-D-leucyl)amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentaazaoctacyclo[26.14.2.2.2.1.1.0.0]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34,3 
6,38,46,49-pentadecaene-40-carboxylic acid
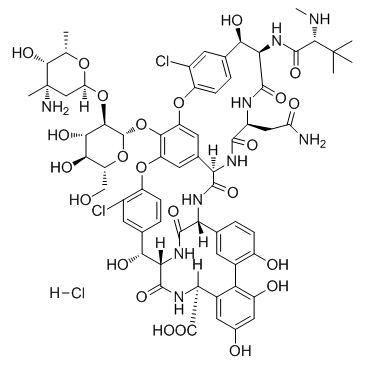
(1S,2R,18R,19R,22S,25R,28R,40S)-22-(2-amino-2-oxoethyl)-48-{[2-O-(3-amino-2,3,6-trideoxy-3-methyl-α-L-lyxo-hexopyranosyl)-β-D-glucopyranosyl]oxy}-5,15-dichloro-2,18,32,35,37-pentahydroxy-19-[(N-methyl-D-leucyl)amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentaazaoctacyclo[26.14.2.2.2.1.1.0.0]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34,36,38,46,49-pentadecaene-40-carboxylic acid Vancocin Vancomycin HCL (1S,2R,18R,19R,22S,25R,28R,40S)-22-(2-Amino-2-oxoethyl)-48-{[(5ξ)-2-O-(3-amino-2,3,6-trideoxy-3-methyl-α-L-lyxo-hexopyranosyl)-β-D-xylo-hexopyranosyl]oxy}-5,15-dichloro-2,18,32,35,37-pentahydr oxy-19-[(N-methyl-D-leucyl)amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentaazaoctacyclo[26.14.2.2.2.1.1.0.0]pentaconta-3,5,8(48),9,11,14,16,29(45), 30,32,34,36,38,46,49-pentadecaene-40-carboxy Lyphocin (LyphoMed) VANCOR Vancomycin hydrochloride Vancomycin (hydrochloride) EINECS 604-193-8 |
| Description | Vancomycin hydrochloride is an antibiotic for the treatment of bacterial infections. It acts by inhibiting the second stage of cell wall synthesis of susceptible bacteria. Vancomycin also alters the permeability of the cell membrane and selectively inhibits ribonucleic acid synthesis. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1] |
| In Vitro | Vancomycin is a large glycopeptide compound with a molecular weight of 1450 Da[1]. Vancomycin is a unique glycopeptide structurally unrelated to any currently available antibiotic. It also has a unique mode of action inhibiting the second stage of cell wall synthesis of susceptible bacteria. Vancomycin is active against a large number of species of Gram-positive bacteria, such as Staphylococcus aureus, Staph. epidermidis, Str. agalactiae, Str. bovis, Str. mutans, viridans streptococci, enterococci[2]. |
| In Vivo | Vancomycin is administered intravenously, with a standard infusion time of at least 1 h, to minimize infusion-related adverse effects. Subjects with normal creatinine clearance, vancomycin has an α-distribution phase of 30 min to 1 h and a β-elimination half-life of 6-12 h. The volume of distribution is 0.4–1 L/kg. The binding of vancomycin to protein ranges from 10% to 50%. Factors that affect the overall activity of vancomycin include its tissue distribution, inoculum size, and protein-binding effects[1]. Vancomycin treatment of infected mice is associated with improved clinical, diarrhea, and histopathology scores and survival during treatment[3]. |
| Animal Admin | Mice: One set of experiments is performed in which infected mice are treated with vancomycin (50 mg/kg) daily for 1, 2, 3, or 5 days and are observed for 21 days postinfection or with vancomycin (20 mg/kg) daily for either 5 or 10 days and monitoring for 15 days postinfection[3]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Melting Point | >190°C (dec.) |
| Molecular Formula | C66H76Cl3N9O24 |
| Molecular Weight | 1485.714 |
| Flash Point | 87℃ |
| Exact Mass | 1483.406860 |
| PSA | 530.49000 |
| LogP | -1.44 |
| Index of Refraction | 1.735 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 50 mg/mL, clear, yellow | Soluble in water. Slightly soluble in methanol, ethanol and dimethylsulfoxide. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R43 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | YW4380000 |
| HS Code | 2941909000 |
| HS Code | 2941909000 |
|---|