533884-09-2
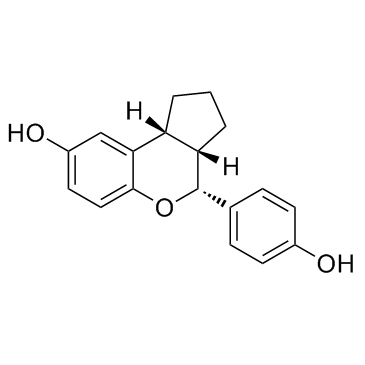
| Name | (3aS,4R,9bR)-4-(4-hydroxyphenyl)-1,2,3,3a,4,9b-hexahydrocyclopenta[c]chromen-8-ol |
|---|---|
| Synonyms |
Erteberel
(3aS,4R,9bR)-4-(4-Hydroxyphenyl)-1,2,3,3a,4,9b-hexahydrocyclopenta[c]chromen-8-ol (3aS,4R,9bR)-1,2,3,3a,4,9b-Hexahydro-4-(4-hydroxyphenyl)cyclopenta[c][1]benzopyran-8-ol LY500307 |
| Description | Erteberel (LY500307) is a potent and selective estrogen receptor beta (ERβ) inhibitor with Ki and EC50 of 1.54 nM and 3.61 nM, respectively[1]. Anti-tumor activities[2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 1.54 nM (ERβ)[1] EC50: 3.61 nM[1] |
| In Vitro | Treatment with LY500307 significantly reduces the proliferation of GBM cells with no activity on normal astrocytes in vitro[2]. ERβ agonists promote apoptosis of GBM cells. It modulated several pathways related to apoptosis, cell cycle, and DNA damage response[2]. LY500307 sensitizes GBM cells to several FDA-approved chemotherapeutic drugs including cisplatin, lomustine and temozolomide[2]. |
| In Vivo | LY500307 treatment significantly reduces tumor growth and promotes apoptosis of GBM tumors in an orthotopic model[2]. LY500307 treatment improves the overall survival of tumor-bearing mice in the GL26 syngeneic glioma model[2]. |
| References |
| Density | 1.268 |
|---|---|
| Boiling Point | 485.2±45.0 °C at 760 mmHg |
| Molecular Formula | C18H18O3 |
| Molecular Weight | 282.33 |
| Flash Point | 247.3±28.7 °C |
| Exact Mass | 282.125580 |
| PSA | 49.69000 |
| LogP | 3.84 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.639 |