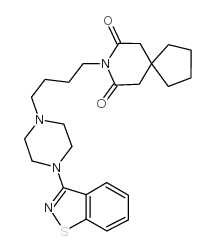
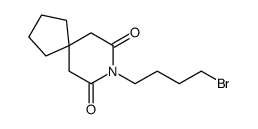
87691-91-6
| Name | 8-[4-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]butyl]-8-azaspiro[4.5]decane-7,9-dione |
|---|---|
| Synonyms |
Tiospirona [Spanish]
tiaspirone Tiospirone [INN] Tiospironum [Latin] [14C]-Tiospirone Tiospironum Tiospirona 8-{4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]butyl}-8-azaspiro[4.5]decane-7,9-dione 8-Azaspiro(4,5)decane-7,9-dione,8-(4-(4-(1,2-benzisothiazol-3-yl)-1-piperazinyl)butyl) TIOSPIRONE |
| Description | Tiospirone is a 5-HT2 receptor antagonist with affinity for D2, 5-HT1a, and 5-HT7, and sigma receptors. Tiospirone decreases consumption of ethanol while increasing food intake of rats. Tiospirone can also reduce the reinforcing properties of Cocaine exhibited in the conditioned place preference paradigm[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.29g/cm3 |
|---|---|
| Boiling Point | 600.9ºC at 760 mmHg |
| Molecular Formula | C24H32N4O2S |
| Molecular Weight | 440.60100 |
| Flash Point | 317.2ºC |
| Exact Mass | 440.22500 |
| PSA | 84.99000 |
| LogP | 3.84870 |
| Index of Refraction | 1.652 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |