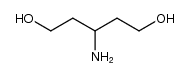
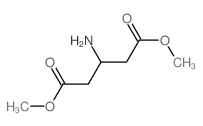
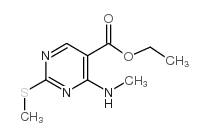
449811-01-2
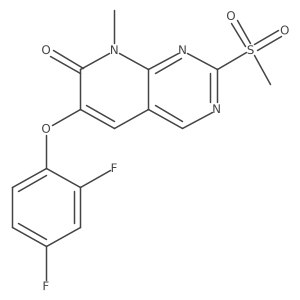
| Name | 6-(2,4-difluorophenoxy)-2-(1,5-dihydroxypentan-3-ylamino)-8-methylpyrido[2,3-d]pyrimidin-7-one |
|---|---|
| Synonyms |
Pamapimod
R-1503 |
| Description | Pamapimod is a novel p38 mitogen-activated protein kinase inhibitor. Pamapimod inhibited p38α and p38β enzymatic activity, with IC50 values of 0.014 ± 0.002 and 0.48 ± 0.04 μM, respectively. Pamapimod is p38 inhibitor with IC50 of 0.06μM in THP-1 cell.IC50: 0.014 ± 0.002 and 0.48 ± 0.04 μM(p38α and p38β enzymatic activity,respectively.)IC50:0.06μM (THP-1 cell)[1]In vitro: Pamapimod inhibited p38α and p38β enzymatic activity, with IC50 values of 0.014 ± 0.002 and 0.48 ± 0.04 μM, respectively. There was no activity against p38δ or p38γ isoforms. When profiled across 350 kinases, pamapimod bound only to four kinases in addition to p38. Cellular potency was assessed using phosphorylation of heat shock protein-27 and c-Jun as selective readouts for p38 and c-Jun NH2-terminal kinase (JNK), respectively. Pamapimod inhibited p38 (IC50, 0.06 μM), but inhibition of JNK was not detected.Pamapimod also inhibited lipopolysaccharide (LPS)-stimulated tumor necrosis factor (TNF) α production by monocytes, interleukin (IL)-1β production in human whole blood, and spontaneous TNFα production by synovial explants from RA patients. LPS- and TNFα-stimulated production of TNFα and IL-6 in rodents also was inhibited by pamapimod. [1]In vivo: In murine collagen-induced arthritis, pamapimod reduced clinical signs of inflammation and bone loss at 50 mg/kg or greater. In a rat model of hyperalgesia, pamapimod increased tolerance to pressure in a dose-dependent manner, suggesting an important role of p38 in pain associated with inflammation. Finally, an analog of pamapimod that has equivalent potency and selectivity inhibited renal disease in lupus-prone MRL/lpr mice. [1] |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C19H20F2N4O4 |
|---|---|
| Molecular Weight | 406.38300 |
| Exact Mass | 406.14500 |
| PSA | 112.73000 |
| LogP | 1.36620 |
| Storage condition | -20℃ |
|
~91% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
|
~% 
449811-01-2 |
| Literature: Goldstein, David M.; Soth, Michael; Gabriel, Tobias; Dewdney, Nolan; Kuglstatter, Andreas; Arzeno, Humberto; Chen, Jeffrey; Bingenheimer, William; Dalrymple, Stacie A.; Dunn, James; Farrell, Robert; Frauchiger, Sandra; La Fargue, Joann; Ghate, Manjiri; Graves, Bradford; Hill, Ronald J.; Li, Fujun; Litman, Renee; Loe, Brad; McIntosh, Joel; McWeeney, Daniel; Papp, Eva; Park, Jaehyeon; Reese, Harlan F.; Roberts, Richard T.; Rotstein, David; San Pablo, Bong; Sarma, Keshab; Stahl, Martin; Sung, Man-Ling; Suttman, Rebecca T.; Sjogren, Eric B.; Tan, Yunchou; Trejo, Alejandra; Welch, Mary; Weller, Paul; Wong, Brian R.; Zecic, Hasim Journal of Medicinal Chemistry, 2011 , vol. 54, # 7 p. 2255 - 2265 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |