4176-97-0
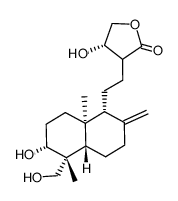
| Name | 3-{2-[(1R,4aS,5R,6R,8aS)-6-Hydroxy-5-(hydroxymethyl)-5,8a-dimethy l-2-methylenedecahydro-1-naphthalenyl]ethyl}-2(5H)-furanone |
|---|---|
| Synonyms |
20-ethyl-7,8-dihydroxy-1,6,16-trimethoxy-4-(methoxymethyl)aconitan-14-one
14-Dehydro-browniin 14-Dehydrobrowniine Aconitan-14-one,20-ethyl-7,8-dihydroxy-1,6,16-trimethoxy-4-(methoxymethyl)-,(1alpha,6beta,16beta) 14-deoxy andrographolide Dehydrobrowniin 10-Dehydro-browniin 3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxyl-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethyl)furan-2(5H)-one Deoxyandrographolide |
| Description | 14-Deoxyandrographolide, a bioactive compound of Andrographis paniculata, has hepatoprotective efficacy. 14-Deoxyandrographolide desensitizes hepatocytes to TNF-α-mediated apoptosis through the release of TNFRSF1A release[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.15g/cm3 |
|---|---|
| Boiling Point | 509.5ºC at 760 mmHg |
| Melting Point | 176-178ºC |
| Molecular Formula | C20H30O4 |
| Molecular Weight | 334.45000 |
| Flash Point | 177.3ºC |
| Exact Mass | 334.21400 |
| PSA | 66.76000 |
| LogP | 2.99180 |
| Index of Refraction | 1.551 |
| Storage condition | 2-8℃ |
|
~82% 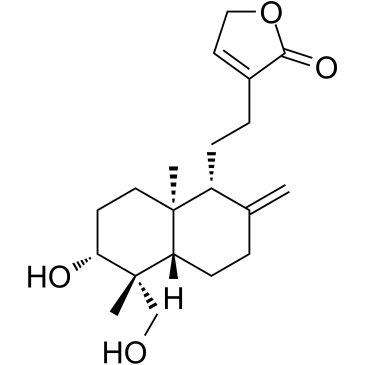
4176-97-0 |
| Literature: Pandeti, Sukanya; Sonkar, Ravi; Shukla, Astha; Bhatia, Gitika; Tadigoppula, Narender European Journal of Medicinal Chemistry, 2013 , vol. 69, p. 439 - 448 |
|
~% 
4176-97-0 |
| Literature: Pal, Mahesh; Singh, Meenakshi; Sharma Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2002 , vol. 41, # 9 p. 1915 - 1918 |
|
~2% 
4176-97-0 |
| Literature: Fujita, Tetsuro; Fujitani, Ryujiro; Takeda, Yoshio; Takaishi, Yoshihisa; Yamada, Toshihide; et al Chemical & Pharmaceutical Bulletin, 1984 , vol. 32, # 6 p. 2117 - 2125 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |