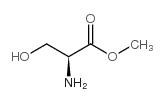
82911-78-2
| Name | (S)-Methyl 2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-hydroxypropanoate |
|---|---|
| Synonyms |
Serine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-, methyl ester
Methyl N-[(9H-fluoren-9-ylmethoxy)carbonyl]serinate methyl (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-hydroxypropanoate Fmoc-Ser-Ome |
| Description | Fmoc-Ser-OMe (Fmoc-L-Ser-OMe) is a hydroxylated L-amino acid protected with a 9-fluorenylmethyloxycarbonyl (Fmoc) group. Fmoc-Ser-OMe involves in chlorophyll–amino acid conjugates synthesis, and acts as a chromo/fluorophores modified protein and emits visible to near-infrared lights efficiently. Fmoc-Ser-OMe glycosylates and produces small mucin-related Olinked glycopeptides, as an alcohol acceptor[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 579.4±45.0 °C at 760 mmHg |
| Molecular Formula | C19H19NO5 |
| Molecular Weight | 341.358 |
| Flash Point | 304.2±28.7 °C |
| Exact Mass | 341.126312 |
| PSA | 88.35000 |
| LogP | 3.25 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.595 |
| Storage condition | -15°C |
| HS Code | 2924299090 |
|---|
|
~98% 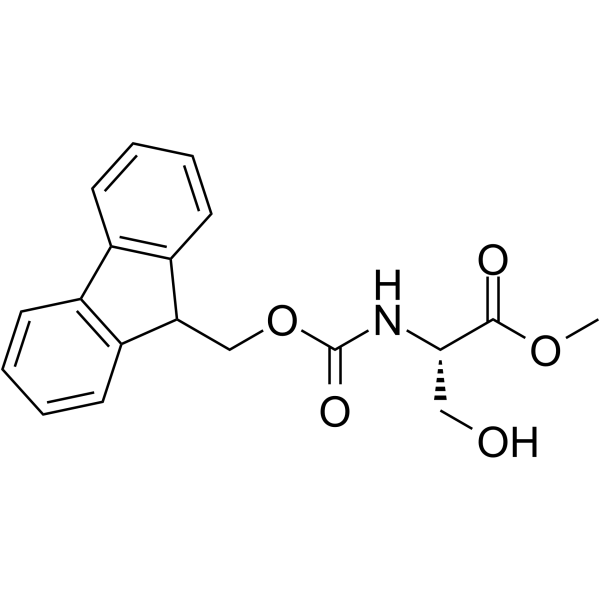
82911-78-2 |
| Literature: Shao, Hui; Lockman, Jeffrey W.; Parquette, Jon R. Journal of the American Chemical Society, 2007 , vol. 129, # 7 p. 1884 - 1885 |
|
~94% 
82911-78-2 |
| Literature: Zhang, Fa; Zhang, Wei; Zhang, Yan; Curran, Dennis P.; Liu, Gang Journal of Organic Chemistry, 2009 , vol. 74, # 6 p. 2594 - 2597 |
|
~% 
82911-78-2 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 22, # 4 p. 1421 - 1428 |
|
~80% 
82911-78-2 |
| Literature: Lapatsanis, Lucas; Milias, George; Froussios, Kleanthis; Kolovos, Miltiadis Synthesis, 1983 , # 8 p. 671 - 673 |
|
~% 
82911-78-2 |
| Literature: Organic letters, , vol. 3, # 16 p. 2477 - 2479 |
|
~% 
82911-78-2 |
| Literature: Canadian Journal of Chemistry, , vol. 60, p. 976 - 980 |
|
~% 
82911-78-2 |
| Literature: Canadian Journal of Chemistry, , vol. 60, p. 976 - 980 |
|
~% 
82911-78-2 |
| Literature: Canadian Journal of Chemistry, , vol. 60, p. 976 - 980 |
| Precursor 10 | |
|---|---|
| DownStream 5 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |





![2-PYRIDINEPROPANOIC ACID, 5-BROMO-.ALPHA.-[[(9H-FLUOREN-9-YLMETHOXY)CARBONYL]AMINO]-, (.ALPHA.S) structure](https://image.chemsrc.com/caspic/474/282734-37-6.png)


