71678-03-0
| Name | 3-[[(1R,2S,4aS,8aS)-1,2,4a-trimethyl-5-methylidene-3,4,6,7,8,8a-hexahydro-2H-naphthalen-1-yl]methyl]-2-hydroxy-5-methoxycyclohexa-2,5-diene-1,4-dione |
|---|---|
| Synonyms |
2-Hydroxy-5-methoxy-3-{[(1R,2S,4aS,8aS)-1,2,4a-trimethyl-5-methylenedecahydro-1-naphthalenyl]methyl}-1,4-benzoquinone
Illimaquinone Ilimaquinone Imaquinone 2-hydroxy-5-methoxy-3-{[(1R,2S,4aS,8aS)-1,2,4a-trimethyl-5-methylidenedecahydronaphthalen-1-yl]methyl}cyclohexa-2,5-diene-1,4-dione MFCD00274432 |
| Description | Ilimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway. Ilimaquinone can induce vesiculation of the Golgi apparatus[1]. Ilimaquinone exerts anti-HIV, anti-microbial, anti-inflammatory, and effects[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Ilimaquinone induces a concentration-dependent anti-proliferative effect in several types of cancer cell lines, including prostate cancer PC-3 and LNCaP, non-small cell lung cancer A549 and hepatocellular carcinoma Hep3B cells. Ilimaquinone (0.3-30 μM; 48 hours) inhibits the proliferation of PC-3 cells, DU145, LNCaP, MG63, A549, Hep3B cells with GI50s of 2.6 μM, 5.8, 4.6, 4.9, 4.1, 12.0μM, respectively[1]. Cell Viability Assay[1] Cell Line: Prostate cancer PC-3 cells Concentration: 0.3, 1, 3, 10, and 30 μM Incubation Time: 48 hours Result: Inhibited the proliferation of PC-3 cells in a concentration-dependent manner. |
| In Vivo | Ilimaquinone exhibits terminal elimination half-lives (T1/2=1.2±0.3 h) due to high plasma clearance (2.95±0.53 L/h/kg) following oral administration (10 mg/kg) in male Sprague-Dawley rats[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 478.4±45.0 °C at 760 mmHg |
| Molecular Formula | C22H30O4 |
| Molecular Weight | 358.471 |
| Flash Point | 159.9±22.2 °C |
| Exact Mass | 358.214417 |
| PSA | 63.60000 |
| LogP | 6.38 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.549 |
| WGK Germany | 3 |
|---|
| Precursor 8 | |
|---|---|
| DownStream 1 | |
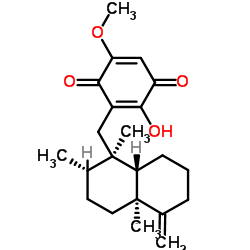
![3-[[(1R,2S,4aS,8aS)-1,2,4a-trimethyl-5-methylidene-3,4,6,7,8,8a-hexahydro-2H-naphthalen-1-yl]methyl]-2,5-dimethoxycyclohexa-2,5-diene-1,4-dione structure](https://image.chemsrc.com/caspic/316/121994-56-7.png)


![(1R,4aS)-trans-decahydro-1-α-[(2,3,5,6-tetramethoxyphenyl)methyl]-1β,4aβ-dimethyl-2-methylene-5-(2-methyl-1,3-dioxolan-2-yl)-naphthalene structure](https://image.chemsrc.com/caspic/204/213026-15-4.png)
![(1R,2S,4aS)-trans-decahydro-1α-[(2,3,5,6-tetramethoxyphenyl)methyl]-1β,2β,4aβ-trimethyl-5-methylene-naphthalene structure](https://image.chemsrc.com/caspic/090/213026-18-7.png)