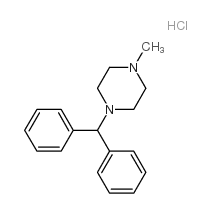
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TL6560000
-
CHEMICAL NAME :
-
Piperazine, 1-(diphenylmethyl)-4-methyl-, hydrochloride
-
CAS REGISTRY NUMBER :
-
303-25-3
-
LAST UPDATED :
-
198910
-
DATA ITEMS CITED :
-
13
-
MOLECULAR FORMULA :
-
C18-H22-N2.Cl-H
-
MOLECULAR WEIGHT :
-
302.88
-
WISWESSER LINE NOTATION :
-
T6N DNTJ A1 DYR&R &GH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
165 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 112,297,1954
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
58 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PHMGBN Pharmacology: International Journal of Experimental and Clinical Pharmacology. (S. Karger AG, Postfach CH-4009 Basel, Switzerland) V.1- 1968- Volume(issue)/page/year: 13,241,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Bird - pigeon
-
DOSE/DURATION :
-
106 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
JAPMA8 Journal of the American Pharmaceutical Association, Scientific Edition. (Washington, DC) V.29-49, 1940-60. For publisher information, see JPMSAE. Volume(issue)/page/year: 46,140,1957 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
225 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 12-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 147,391,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
525 mg/kg
-
SEX/DURATION :
-
female 6-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 7-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Fertility - other measures of fertility Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
525 mg/kg
-
SEX/DURATION :
-
female 6-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
525 mg/kg
-
SEX/DURATION :
-
female 6-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - other measures of fertility Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 7-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - other measures of fertility Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 1-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
350 mg/kg
-
SEX/DURATION :
-
female 8-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
525 mg/kg
-
SEX/DURATION :
-
female 8-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
COREAF Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. (Paris, France) V.1-261, 1835-1965. For publisher information, see CRASEV. Volume(issue)/page/year: 256,3359,1963
|