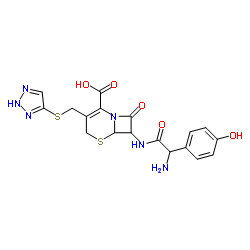
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XI0340000
-
CHEMICAL NAME :
-
5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-((amino(4-hydroxyphenyl)acetyl) amino)-8-oxo-3-((1H-1,2,3-triazol-4-ylthio)methyl)-, (6R-(6-alpha,7-beta(R)))-
-
CAS REGISTRY NUMBER :
-
51627-14-6
-
LAST UPDATED :
-
199410
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C18-H18-N6-O5-S2
-
MOLECULAR WEIGHT :
-
462.54
-
WISWESSER LINE NOTATION :
-
T46 ANV ES GUTJ CMVYZR DQ& HVQ G1S- DT5MNNJ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>6 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4325 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Skin and Appendages - hair
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>6 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - changes in structure or function of salivary glands
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>240 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>6 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6410 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Skin and Appendages - hair
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>6 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - changes in structure or function of salivary glands
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1600 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>40 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Skin and Appendages - hair
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>80 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,612,1976 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
175 mg/kg
-
SEX/DURATION :
-
female 8-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,129,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2800 mg/kg
-
SEX/DURATION :
-
female 8-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,129,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5600 mg/kg
-
SEX/DURATION :
-
female 8-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,129,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
175 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,129,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
225 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,144,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3600 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 29,144,1976
|