533-45-9
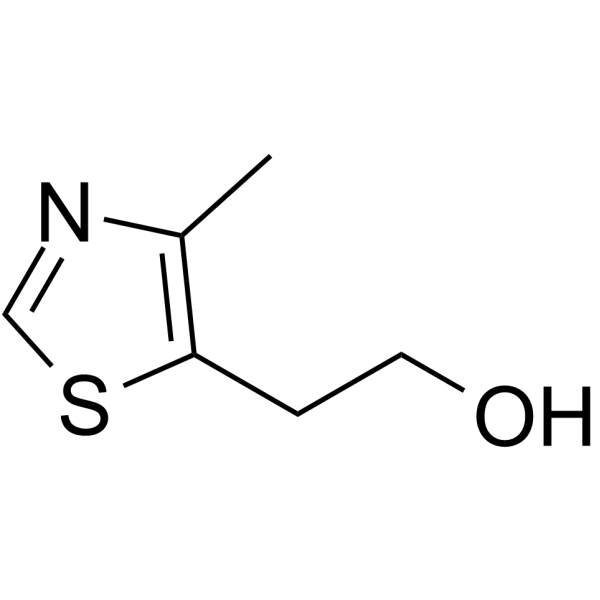
| Name | 5-(2-chloroethyl)-4-methyl-1,3-thiazole |
|---|---|
| Synonyms |
Clomethiazole
Emineurina Clomethiazol Chlormethiazole MFCD00868012 Distraneurin Clometiazole Chlormethiazol Clomethiazolum EINECS 208-565-7 |
| Description | Chlormethiazole is an potent and orally active GABAA agonist[1]. Chlormethiazole inhibits cytochrome P450 isoforms: CYP2A6 and CYP2E1 in human liver microsomes. Chlormethiazole is an anticonvulsant agent and has the potential for treating convulsive status epilepticus[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Clomethiazole (1 mM), in the absence of GABA, to α1/β1/γ2 or α1/β2/γ2 subunit-containing cells produced large whole-cell currents[1]. Clomethiazole activate GABAA currents in α1/β1/γ2- and α1/β2/γ2-containing cells, with EC50 values of 0.3 and 1.5 mM, respectively[1]. Clomethiazole (30 μM) at low concentration also potentiates the action of GABA in both cell types, equivalent to a 3-fold increase in potency and up to 1.8-fold increase in maximal current[1]. Clomethiazole inhibits cytochrome P450 isoforms, CYP2A6 and CYP2E1 with IC50 values of 24 µM and 42 µM, respectively, in human liver microsomes, meanwhile all other isoforms exhibiting values > 300 µM[2]. |
| References |
| Density | 1.218 g/cm3 |
|---|---|
| Boiling Point | 245.8ºC at 760 mmHg |
| Molecular Formula | C6H8ClNS |
| Molecular Weight | 198.11300 |
| Flash Point | 102.4ºC |
| Exact Mass | 196.98300 |
| PSA | 41.13000 |
| LogP | 3.03480 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2934100090 |
|---|
|
~96% 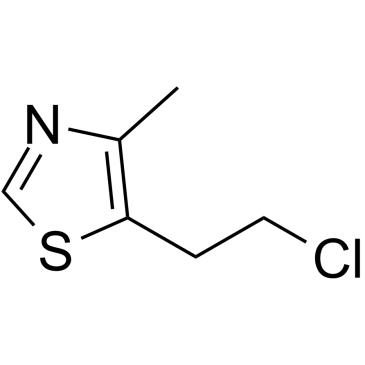
533-45-9 |
| Literature: Van den Hoven; Alper Journal of the American Chemical Society, 2001 , vol. 123, # 6 p. 1017 - 1022 |
|
~% 
533-45-9 |
| Literature: Journal of the American Chemical Society, , vol. 57, p. 1879 |
|
~% 
533-45-9 |
| Literature: Journal of the American Chemical Society, , vol. 57, p. 1879 |
|
~% 
533-45-9 |
| Literature: Journal of the American Chemical Society, , vol. 57, p. 1879 |
|
~% 
533-45-9 |
| Literature: DE678153 , ; DRP/DRBP Org.Chem. |
|
~% 
533-45-9 |
| Literature: Journal of the American Chemical Society, , vol. 58, p. 1803 |
| Precursor 5 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |