50-55-5
| Name | reserpine |
|---|---|
| Synonyms |
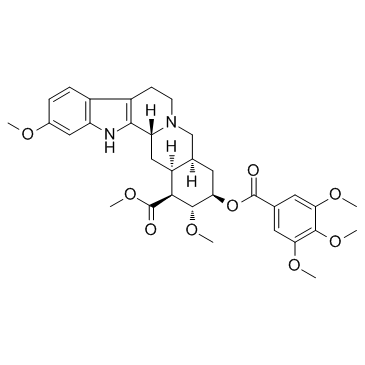
methyl (1S,2R,3R,4aS,13bR,14aS)-2,11-dimethoxy-3-{[(3,4,5-trimethoxyphenyl)carbonyl]oxy}-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2',3':3,4]pyrido[1,2-b]isoquinoline-1-carboxylate
methyl (1R,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-(3,4,5-trimethoxybenzoyl)oxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate MFCD00005091 Hiserpia Methyl 18b-Hydroxy-11,17a-dimethoxy-3b,20a-yohimban-16b-carboxylate 3,4,5-Trimethoxybenzoate (Ester) Serpipur EINECS 200-047-9 Raunervil Serpanray Methyl (3β,16β,17α,18β,20α)-11,17-dimethoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]yohimban-16-carboxylate Methyl-(1S,2R,3R,4aS,13bR,14aS)-2,11-dimethoxy-3-{[(3,4,5-trimethoxyphenyl)carbonyl]oxy}-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2',3':3,4]pyrido[1,2-b]isochinolin-1-carboxylat methyl reserpate 3,4,5-trimethoxybenzoic acid ester Raupasil SERPASIL Rausedil rivasin reserpine [3H]-Reserpine Serpivite (-)-reserpine Reserpine Base serpentina Serp-AFD Serpalan Apoplon |
| Description | Reserpine is an inhibitor of the vesicular monoamine transporter 2 (VMAT2). |
|---|---|
| Related Catalog | |
| Target |
VMAT2[1] |
| In Vitro | Reserpine is an inhibitor of the vesicular monoamine transporter 2 (VMAT2). Reserpine displays a significant effect on the density of dopamine D1 receptors (F2,12=8.81, p<0.01) in the rat striatum. The affinity (Kd) for the dopamine D1 and D2 receptors during withdrawal from acute and chronic administration of reserpine is not change[1]. IC50 values of 43.9 and 54.9 μM are obtained after 1 day of treatment with Reserpine in JB6 P+ and HepG2-C8 cells, respectively. Reserpine induces luciferase activity in a dose-dependent manner at concentrations ranging from 5 to 50 μM, and no significant induction is observed at concentrations lower than 5 μM. Results demonstrate that Reserpine (2.5 to 10 μM) also increases the protein expression of Nrf2, HO-1, and NQO1. Reserpine at concentrations of 2.5 to 10 μM decreases the mRNA expression of DNMT1, DNMT3a, and DNMT3b in a concentration-dependent manner in JB6 P+ cells after 7 days of treatment. Reserpine at 10 μM generates a significant difference for DNMT3a expression (p<0.05)[2]. |
| In Vivo | Withdrawal (48 h) from chronic (14-day) but not acute Reserpine administration in a dose of 0.2 mg/kg i.p. produces a significant reduction of the immobility time (F2,18=3.68, p<0.05), but increases the climbing time (F2,18=4.48, p<0.02), and does not change the swimming time (F2,18=1.78; NS) in the forced swim test (FST) in rats[1]. Reserpine at a dose of 5 mg/kg body weight produces significant increase in the urinary excretion profile of vanillylmandelic acid (VMA) compare to control animals. The amount of 5-hydroxyindoleacetic acid (5-HIAA) excreted in animals treated with Reserpine is found to be more than in the control. Dose dependent hypotension is observed with Reserpine. Reserpine at doses of 0.5, 1, 5, 10 and 15 μg/kg produce significant (p<0.01) reduction in blood pressure compare to control[3]. |
| Kinase Assay | After incubation for 24 h, JB6 P+ cells (1×105 cells/10-cm dish) are treated with various concentrations of Reserpine. Whole cell lysates are prepared from the treated cells using radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail, and a BCA kit is used to determine protein concentrations[2]. |
| Cell Assay | JB6 P+ cells are seeded in 96-well plates containing Minimum essential media (MEM) at a density of 1×104 cells/mL (100 μL/well) for 1, 3, and 5 days, and HepG2-C8 cells are seeded in plates containing DMEM. After incubation for 24 h, the cells are treated with either DMSO or various concentrations of Reserpine. For JB6 P+ cells, the medium is changed every 2 days for the 3-day and 5-day treatments. Cell viability is assessed using a MTS assay kit according to the manufacturer’s instructions. The absorbance of the formazan product is read at 490 nm, and the cell viability is calculated and compared with the DMSO control group[2]. |
| Animal Admin | Albino rats of either sex weighing between 100 to 150 g are used in the study. They are acclimatized to the laboratory conditions for at least 10 days prior to the experiment and provided with standard diet and water ad libitum with 12 h light and dark cycle. Animals are divided into different groups of six each and are housed individually in metabolic cages. Group 1: Control animals treated with DMSO intraperitoneally at a dose of 0.1 mL/100 g body weight. Group 2: Animals administered intraperitoneally with Reserpine at a dose of 5 mg/kg body weight. The 24 h urine samples from the point of drug administration are collected for each animal[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 700.1±60.0 °C at 760 mmHg |
| Melting Point | 265ºC (dec.) |
| Molecular Formula | C33H40N2O9 |
| Molecular Weight | 608.679 |
| Flash Point | 377.2±32.9 °C |
| Exact Mass | 608.273376 |
| PSA | 117.78000 |
| LogP | 4.05 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.620 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R10;R36;R67 |
| Safety Phrases | S26 |
| RIDADR | UN 1219 |
| WGK Germany | 3 |
| RTECS | ZG0350000 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 3003909090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 3003909090 |
|---|