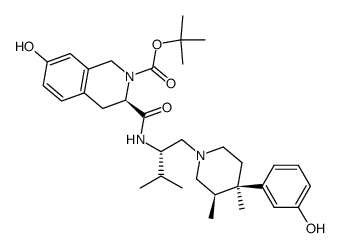
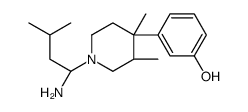
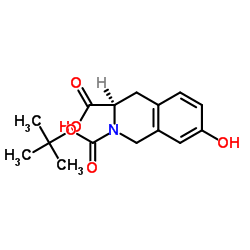
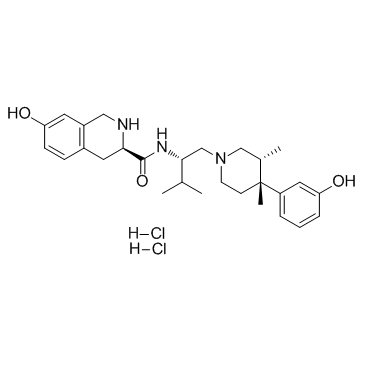
785835-79-2
| Name | (3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide dihydrochloride |
|---|---|
| Synonyms |
(3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide
JDTic dihydrochloride JDTic 2HCl| 1,2,3,4-tetrahydro-7-hydroxy-N-[(1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl]-(3R)-isoquinolinecarboxamide JDTic (dihydrochloride) |
| Description | JDTic (dihydrochloride) is a potent antagonist of kappa-opioid receptors (KOR), blocking the κ-agonist U50, 488-induced antinociception. |
|---|---|
| Related Catalog | |
| In Vivo | JDTic (2.5-16 mg/kg, s.c.) dose-dependently blocks the antinociceptive response of nicotine in the tail-flick test but has no effect in the hot-plate assay or body temperature assessments at any dose tested in the mice injected with nicotine[1]. JDTic (3 mg/kg, i.p.) is capable of reversing anxiety-like behavior in the rat model of hangover anxiety. JDTic (10 mg/kg, i.p.) decreases alcohol self-administration, suppresses cue-induced reinstatement of alcohol seeking, and specifically blocks the effects of a KOR agonist at the 2 h pretreatment time point[2]. JDTic (30 mg/kg, i.g.) significantly blockes U50,488-induced diuresis immediately in rats[3]. |
| Animal Admin | Mice: Naive mice are injected s.c. with JDTic (1, 4, 8, or 16 mg/kg) 18 h prior to nicotine (2.5 mg/kg, s.c.). Due to JDTic’s very long duration of action, an 18-h preinjection is chosen for the studies. Antinociception using the tail-flick and hot-plate tests is measured 5 min after nicotine injection or 20 min after morphine (8 mg/kg, s.c.), and changes in body temperature are measured 30 min after injection. To confirm an absence of mu antagonist effects by JDTic in these studies, JDTic (16 mg/kg) is also administered 1, 6, 18, and 24 h before morphine (8 mg/kg, s.c.) in the tail-flick test, and antinociception is measured 20 min after morphine. |
| References |
| Molecular Formula | C28H41Cl2N3O3 |
|---|---|
| Molecular Weight | 538.54900 |
| Exact Mass | 537.25200 |
| PSA | 88.32000 |
| LogP | 6.26360 |
| Storage condition | 2-8℃ |
|
~% 
785835-79-2 |
| Literature: Thomas; Atkinson; Rothman; Fix; Mascarella; Vinson; Xu; Dersch; Lu; Cantrell; Zimmerman; Carroll Journal of Medicinal Chemistry, 2001 , vol. 44, # 17 p. 2687 - 2690 |
|
~% 
785835-79-2 |
| Literature: Thomas, James B.; Atkinson, Robert N.; Vinson, N. Ariane; Catanzaro, Jennifer L.; Perretta, Carin L.; Fix, Scott E.; Mascarella, S. Wayne; Rothman, Richard B.; Xu, Heng; Dersch, Christina M.; Cantrell, Buddy E.; Zimmerman, Dennis M.; Carroll, F. Ivy Journal of Medicinal Chemistry, 2003 , vol. 46, # 14 p. 3127 - 3137 |
|
~% 
785835-79-2 |
| Literature: Thomas, James B.; Atkinson, Robert N.; Vinson, N. Ariane; Catanzaro, Jennifer L.; Perretta, Carin L.; Fix, Scott E.; Mascarella, S. Wayne; Rothman, Richard B.; Xu, Heng; Dersch, Christina M.; Cantrell, Buddy E.; Zimmerman, Dennis M.; Carroll, F. Ivy Journal of Medicinal Chemistry, 2003 , vol. 46, # 14 p. 3127 - 3137 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |