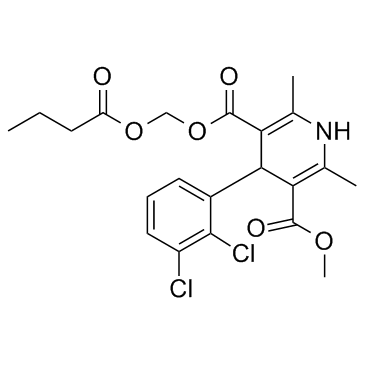
167221-71-8
| Name | 5-O-(butanoyloxymethyl) 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate |
|---|---|
| Synonyms |
(butanoyloxy)methyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
Cleviprex clevidipine (±)-Hydroxymethyl Methyl 4-(2,3-Dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate Butyrate (Ester) Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate Clevidipine butyrate Clevidipine,Cleviprex H 324/38 (Butyryloxy)methyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate Clevelox 3,5-Pyridinedicarboxylic acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, methyl (1-oxobutoxy)methyl ester rac-Clevidipine |
| Description | Clevidipine is a short-acting dihydropyridine calcium channel antagonist (IC50= 7.1 nM, V(H) = -40 mV ) under development for treatment of perioperative hypertension.IC50 Value: 7.1 nM at V(H) = -40 mV [1]Target: calcium channelin vitro: Both clevidipine and nitroglycerin completely reversed U46619-induced contraction (clevidipine (50% effective concentration [EC50] = 3.88 +/- 0.84 x 10(-6) mol/L, nitroglycerin EC50 = 4.84 +/- 2.76 x 10(-8) mol/L) [2]. A decrease in temperature increased the half-life of clevidipine in blood, whereas dilution of the blood did not affect the in vitro half-life of clevidipine. The albumin concentration affected the hydrolysis rate of clevidipine in RBC suspended with saline [3].in vivo: Clevidipine is a high-clearance drug with a relatively small volume of distribution, resulting in an extremely short half-life in all species studied. The median initial half-life of the individual value (Bayesian estimates) is 12, 20, and 22 s in the rabbit, rat, and dog, respectively [4]. The extremely high clearance value and the small volume of distribution resulted in short half-lives of clevidipine, 2.2 and 16.8 min, respectively. The blood concentration and dose rate producing half the maximal effect (i.e. EC50 and ED50) were approximately 25 nM and 1.5 microg/kg/min, respectively [5].Clinical trial: CARVE: Clevidipine for Vasoreactivity Evaluation of the Pulmonary Arterial Bed. Phase 4 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 539.7±50.0 °C at 760 mmHg |
| Melting Point | 128-130°C |
| Molecular Formula | C21H23Cl2NO6 |
| Molecular Weight | 456.316 |
| Flash Point | 280.2±30.1 °C |
| Exact Mass | 455.090240 |
| PSA | 90.93000 |
| LogP | 5.46 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Storage condition | Refrigerator |