24219-97-4
| Name | mianserin |
|---|---|
| Synonyms |
[3H]-(+/-)-Mianserin
Org-4360 (R)-Mianserine Org-5859 MIANSERIN Dibenzoc,fpyrazino1,2-aazepine,1,2,3,4,10,14b-hexahydro-2-methyl (S)-Mianserine 2-methyl-1,2,3,4,10,14b-hexahydro-dibenzo[c,f]pyrazino[1,2-a]azepine |
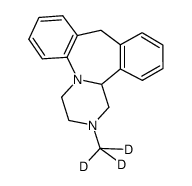
| Description | Mianserin is a H1 receptor inverse agonist and is a psychoactive agent of the tetracyclic antidepressant.Target: H1 receptorMianserin is a psychoactive drug of the tetracyclic antidepressant (TeCA) therapeutic family. It is classified as a noradrenergic and specific serotonergic antidepressant (NaSSA) and has antidepressant, anxiolytic (anti-anxiety), hypnotic (sedating), antiemetic (nausea and vomiting-attenuating), orexigenic (appetite-stimulating), and antihistamine effects. It is not approved for use in the US, but its analogue, mirtazapine, is. Mianserin was the first antidepressant to reach the UK market that was less dangerous than the tricyclic antidepressants in overdose.Mianserin is an antagonist/inverse agonist of the H1, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic, and α2-adrenergic receptors, and also inhibits the reuptake of norepinephrine. As a high affinity H1 receptor inverse agonist, mianserin has strong antihistamine effects (sedation, weight gain, etc.). Contrarily, it has negligible affinity for the mACh receptors, and thus lacks any anticholinergic properties. It was recently found to be a potent kappa opioid receptor agonist. In addition, mianserin also appears to be a potent antagonist of the neuronal octopamine receptor. What implications this may have on mood are currently unknown, however octopamine has been implicated in the regulation of sleep, appetite and insulin production and therefore may theoretically contribute to the overall side effect profile of mianserin. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.18g/cm3 |
|---|---|
| Boiling Point | 411.3ºC at 760mmHg |
| Molecular Formula | C18H20N2 |
| Molecular Weight | 264.36500 |
| Flash Point | 186.1ºC |
| Exact Mass | 264.16300 |
| PSA | 6.48000 |
| LogP | 3.08680 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 3249 |
|---|---|
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~78% 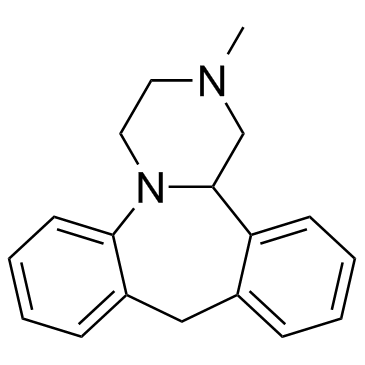
24219-97-4 |
| Literature: Sumitomo Chemical Company, Limited Patent: EP2135870 A1, 2009 ; Location in patent: Page/Page column 5-6 ; |
|
~87% 
24219-97-4 |
| Literature: Hulinska, Hana; Polivka, Zdenek; Jilek, Jiri; Sindelar, Karel; Holubek, Jiri; et al. Collection of Czechoslovak Chemical Communications, 1988 , vol. 53, # 8 p. 1820 - 1844 |
| Precursor 1 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |