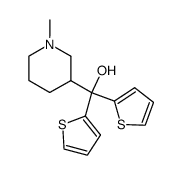
5169-78-8
| Name | 3-(dithiophen-2-ylmethylidene)-1-methylpiperidine |
|---|---|
| Synonyms |
3-(di-thiophen-2-yl-methylene)-1-methyl-piperidine
Bitiodin Asverin (1-Methyl-3-piperidylidene)di(2-thienyl)methane Tipepidine [INN:DCF] Tipepidine 3-(Di-[2]thienyl-methylen)-1-methyl-piperidin Tipepidina [INN-Spanish] Tipedine Tipepidinum [INN-Latin] (1-Methyl-3-piperidyliden)di-2-thienylmethan-embonat CR/662 AT 327 3-(di-[2]thienyl-methylene)-1-methyl-piperidine |
| Description | Tipepidine reversibly inhibits dopamine (DA) D2 receptor-mediated GIRK currents (IDA(GIRK)) with an IC50 of 7.0 μM. Tipepidine subsequently activates VTA dopamine neuron[1]. Tipepidine, a non-narcotic antitussive, exerts an antidepressant-like effect[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 7.0 μM (dopamine D2 receptor)[1] |
| In Vivo | Tipepidine (i.p.; 10-40 mg/kg; 0.5-23 hours) significantly decreases the immobility time in the forced swimming test in ACTH-treated rats. Tipepidine (i.p.; 40 mg/kg) increases the extracellular dopamine level of the nucleus accumbens (NAc) in ACTH-treated rats[2]. Animal Model: Male Wistar rats weighting 150-240 g (5-7 weeks old) [2] Dosage: 10, 20 and 40 mg/kg. Administration: I.p.; 0.5, 5, 23 hours. Result: Decreased the immobility time in the forced swimming test in ACTH-treated rats. |
| References |
| Density | 1.1947 (rough estimate) |
|---|---|
| Boiling Point | bp4-5 178-184° |
| Melting Point | 64-65° |
| Molecular Formula | C15H17NS2 |
| Molecular Weight | 275.43200 |
| Exact Mass | 275.08000 |
| PSA | 59.72000 |
| LogP | 4.27500 |
| Index of Refraction | 1.5300 (estimate) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2934999090 |
|---|
|
~% 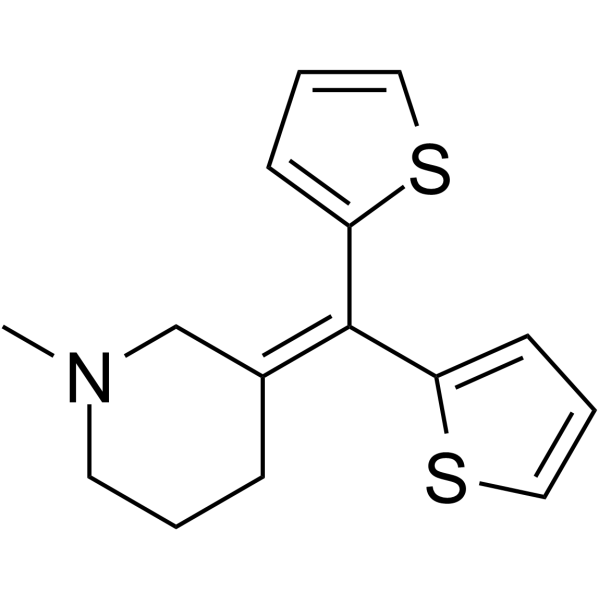
5169-78-8 |
| Literature: Okumura et al. Ann. Rep. Tanabe pharm. Res.Chem.Abstr., 1958 , vol. 3, # 2 p. 30,32 Ann. Rep. Tanabe pharm. Res.Chem.Abstr., 1959 , p. 10214 |
|
~% 
5169-78-8 |
| Literature: Kawazu; Kanno; Saito; Tamaki Journal of medicinal chemistry, 1972 , vol. 15, # 9 p. 914 - 918 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |