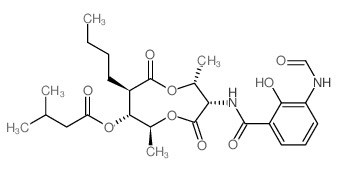
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NY1502900
-
CHEMICAL NAME :
-
Isovaleric acid, N-ester with N-(7-butyl-8-hydroxy-4,9-dimethyl-2,6-dioxo-1,5-dioxo nan-3-yl) -3-formamidosalicylamide, stereoisomer
-
CAS REGISTRY NUMBER :
-
522-70-3
-
LAST UPDATED :
-
199709
-
DATA ITEMS CITED :
-
3
-
MOLECULAR FORMULA :
-
C26-H36-N2-O9
-
MOLECULAR WEIGHT :
-
520.64
-
WISWESSER LINE NOTATION :
-
T9OV EOVTJ CMVR BQ CMVH& D1 G4 HOV1Y1&1 I1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1800 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JAJAAA Journal of Antibiotics, Series A. (Tokyo, Japan) V.6-20, 1953-67. For publisher information, see JANTAJ. Volume(issue)/page/year: 10,39,1957
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1600 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JAJAAA Journal of Antibiotics, Series A. (Tokyo, Japan) V.6-20, 1953-67. For publisher information, see JANTAJ. Volume(issue)/page/year: 10,39,1957
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
900 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85FZAT "Index of Antibiotics from Actinomycetes," Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967 Volume(issue)/page/year: -,145,1967
|