| Description |
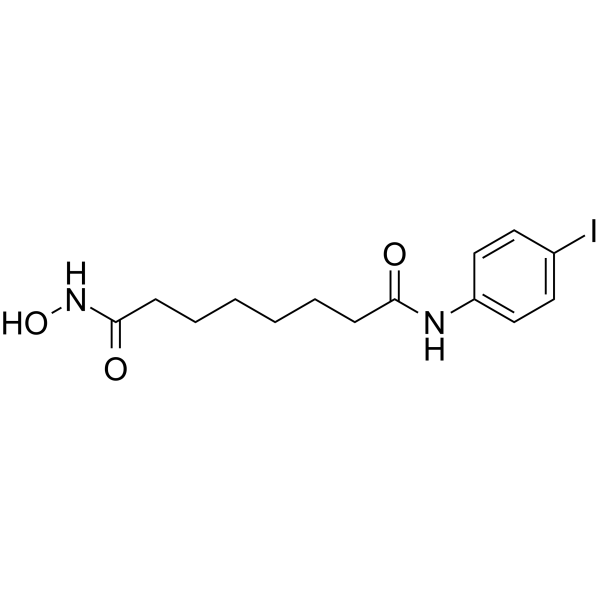
4-Iodo-SAHA (1k) is an orally active class I and class II histone deacetylase (HDAC) inhibitor with EC50s of 1.1, 0.95, 0.12, 0.24, 0.85 and 1.3 μM for Skbr3, HT29, U937, JA16 and HL60 cell lines, respectively. 4-Iodo-SAHA (1k) can be used for the research of cancer[1].
|
| In Vitro |
4-Iodo-SAHA (1k) (0.1-100 μM; 48 h ) inhibits Skbr3, HT29, U937, JA16 and HL60 cell lines [1]. 4-Iodo-SAHA (1k) (2 μM; 6-24 h ) affects acetylated H4 and p21 levels in SKBR3 cells[1]. Cell Proliferation Assay[1] Cell Line: SKBR3, HT29, U937, JA16 and HL60 cell lines Concentration: 0.1-100 μM Incubation Time: 48 h Result: Inhibited SKBR3, HT29, U937, JA16 and HL60 cell lines with EC50s of 1.1, 0.95, 0.12, 0.24, 0.85 and 1.3 μM , respectively. Showed 10-fold potent as an inhibitor of U937 cell line compared to SAHA. Western Blot Analysis[1] Cell Line: SKBR3-breast-derived cell line Concentration: 2 μM Incubation Time: 6, 12 and 24 h Result: Time-dependently up regulated histone H4 acetylation and p21/WAF1 cell cycle inhibitor accumulation in SKBR3 cells.
|