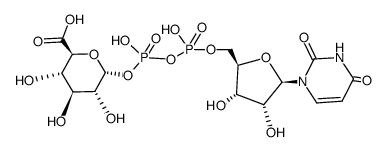
16110-10-4
| Name | acetaminophen O-β-D-glucosiduronic acid |
|---|---|
| Synonyms |
Acetaminophen D-glucuronide
[14C]-Acetaminophen glucuronide paracetamol glucuronide acetaminophen glucuronide acetaminophen O-beta-D-glucosiduronic acid 4-glucuronosido-acetanilide |
| Description | Acetaminophen glucuronide (APAP-glu) is an inactive glucuronide metabolite of Acetaminophen (HY-66005)[1][2]. Acetaminophen is a selective cyclooxygenase-2 (COX-2) inhibitor and a potent hepatic N-acetyltransferase 2 (NAT2) inhibitor[3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Acetaminophen is metabolized in the liver mainly by glucuronidation and sulfation, thus generating the nontoxic metabolites, Acetaminophen glucuronide (APAP-glu). Acetaminophen glucuronide is a substrate for both canalicular Mrp2 and basolateral Mrp3 in rodents[1]. |
| References |
| Density | 1.61g/cm3 |
|---|---|
| Boiling Point | 697.4ºC at 760mmHg |
| Molecular Formula | C14H14D3NO8 |
| Molecular Weight | 330.30500 |
| Flash Point | 375.6ºC |
| Exact Mass | 330.11400 |
| PSA | 145.55000 |
| Vapour Pressure | 2.03E-20mmHg at 25°C |
| Index of Refraction | 1.671 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 29389090 |
|
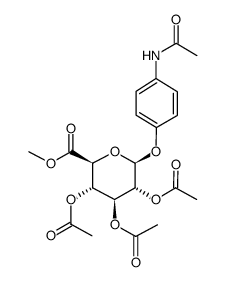
~47% 
16110-10-4 |
| Literature: Hayes, John A.; Eccles, Kevin S.; Lawrence, Simon E.; Moynihan, Humphrey A. Carbohydrate Research, 2012 , vol. 349, p. 108 - 112 |
|
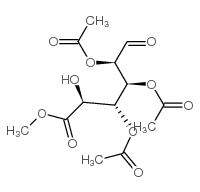
~% 
16110-10-4 |
| Literature: Hayes, John A.; Eccles, Kevin S.; Lawrence, Simon E.; Moynihan, Humphrey A. Carbohydrate Research, 2012 , vol. 349, p. 108 - 112 |
|
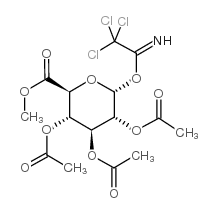
~% 
16110-10-4 |
| Literature: Hayes, John A.; Eccles, Kevin S.; Lawrence, Simon E.; Moynihan, Humphrey A. Carbohydrate Research, 2012 , vol. 349, p. 108 - 112 |
|
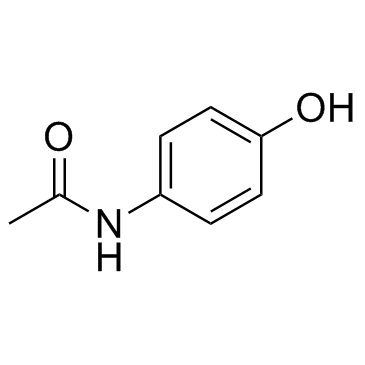
~% 
16110-10-4 |
| Literature: Smith; Williams Biochemical Journal, 1948 , vol. 42, p. 538,541 Biochemical Journal, 1949 , vol. 44, p. 242,245, 250, 251 |
|
~% 
16110-10-4 |
| Literature: Boldt, Petra; Rothschild, Markus A.; Kaeferstein, Herbert Arzneimittel-Forschung/Drug Research, 2007 , vol. 57, # 12 p. 787 - 794 |
|
~% 
16110-10-4 |
| Literature: Wong; Grace Jr.; Wright; Browning; Grossman; Bai; Christ Xenobiotica, 2006 , vol. 36, # 12 p. 1178 - 1190 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |