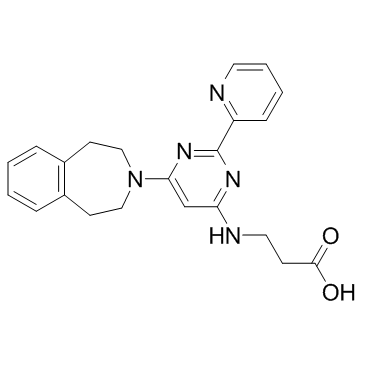
1373422-53-7
| Name | N-[2-(2-Pyridinyl)-6-(1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)-4- pyrimidinyl]-β-alanine |
|---|---|
| Synonyms |
N-[2-(2-Pyridinyl)-6-(1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)-4-pyrimidinyl]-β-alanine
N-[2-(2-methyl-1H-indol-3-yl)ethyl]-4-pyridinecarboxamide GSK-J1 |
| Description | GSK-J1 is a potent inhibitor of H3K27me3/me2-demethylases JMJD3/KDM6B and UTX/KDM6A, with IC50 of 60 nM towards KDM6B. |
|---|---|
| Related Catalog | |
| Target |
IC50: 60 nM (KDM6B)[2] |
| In Vitro | GSK-J1 is selective for H3K27 demethylases of the KDM6 subfamily and specifically binds to endogenous JMJD3. GSK-J1 inhibits TNF-α production by human primary macrophages in an H3K27-dependent manner[1]. GSK-J1 inhibits the demethylase activity of KDM5C with 8.5-fold increased potency compared with that of KDM5B at 1 mM α-ketoglutarate, with IC50 of 11 μM and 94 μM, respectively[3]. |
| Kinase Assay | Purified JmjD3 (1 μM) and UTX (3 μM) is incubated with 10 μM peptide [BiotinKAPRKQLATKAARK(me3 )SAPATGG] in 50 mM HEPES pH 7.5, 150 mM KCl, 50 μM (NH4)2SO4·FeSO4·H2O, 1 mM 2-oxoglutarate, and 2 mM ascorbate (JmjD3, 3 minutes at 25°C; UTX, 20 minutes at 25°C) with various concentration of the inhibitor (0, 0.005, 0.01, 0.02, 0.05, 0.1 μM). 10 mM EDTA is added to stop the reaction. The reaction is desalted by zip tip and spotted on a MALDI plate with α-cyano-4-hydroxycinnamic acid MALDI matrix. Samples are analysed on a MALDI-TOF R system. |
| References |
[2]. Heinemann B, et al. Inhibition of demethylases by GSK-J1/J4. Nature. 2014 Oct 2;514(7520):E1-2. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.9±55.0 °C at 760 mmHg |
| Molecular Formula | C22H23N5O2 |
| Molecular Weight | 389.450 |
| Flash Point | 322.0±31.5 °C |
| Exact Mass | 389.185181 |
| PSA | 91.24000 |
| LogP | 2.75 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.653 |
| Storage condition | -20℃ |
| RIDADR | NONH for all modes of transport |
|---|
| Precursor 3 | |
|---|---|
| DownStream 0 | |